Ambitropin 1200 mg By Cellusyn Laboratories Overview & Drug Interactions
Check For Interactions With Ambitropin 1200 mg
Supplement: Ambitropin 1200 mg by Cellusyn Laboratories
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Vitamin B6
| Ingredient Group | Vitamin B6 |
|---|---|
| Category | vitamin |
- Pyridoxine Hydrochloride
Magnesium
Magnesium is a mineral that is essential for the proper functioning of the body. It plays a role in many important physiological processes, including the contraction and relaxation of muscles, the transmission of nerve impulses, and the regulation of blood pressure. Magnesium is found in a variety of foods, including leafy green vegetables, nuts, and grains, and it is also available as a dietary supplement. There are several different forms of magnesium that are available as supplements, all of which can be used to prevent deficiency. Additionally, magnesium is purported to have several different health benefits, such as improving sleep, reducing muscle cramps, reducing anxiety, and preventing or treating migraines. Although magnesium is essential for health, magnesium-containing foods and supplements can interact with some prescription medications if used at the same time.
See More Information Regarding Magnesium| Ingredient Group | Magnesium |
|---|---|
| Category | mineral |
- Magnesium Aspartate
Zinc
Zinc is a mineral that is essential for the proper functioning of the human body. It is involved in many important physiological processes, including immune system function, wound healing, taste, and smell. Zinc is found in a variety of foods, including meat, seafood, and whole grains, and it is also available as a dietary supplement. Zinc supplements may be used to treat or prevent zinc deficiency, which can occur due to certain medical conditions, such as gastrointestinal disorders, alcohol abuse, and certain medications. Zinc supplements may also be used for other purposes, such as to boost the immune system, improve acne, and reduce the severity and duration of colds. There are several different forms of zinc supplements available, including zinc gluconate, zinc acetate, and zinc sulfate. The most common form of zinc supplements is zinc gluconate, which is well absorbed and is less likely to cause stomach-related side effects than other forms of zinc.
See More Information Regarding Zinc| Ingredient Group | Zinc |
|---|---|
| Category | mineral |
- Zinc Amino Acid Chelate
Valerian
Valerian (Valeriana officinalis) is a perennial herb native to Europe and Asia. The plant is known for its strong, distinctive odor and its purported medicinal effects. Valerian contains a number of active compounds, including valerenic acid and valepotriates, which are believed to have a sedative effect on the body and are may to be helpful in the treatment of insomnia and anxiety. Valerian is also believed to have mild tranquilizing and muscle-relaxing properties and may be helpful in the treatment of muscle spasms and other muscle disorders. The root of the valerian plant is most commonly utilized in dietary supplements and is often standardized for valerenic acid content.
See More Information Regarding Valerian| Ingredient Group | Valerian |
|---|---|
| Category | botanical |
GABA
Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter that is involved in a variety of functions, including regulating brain activity and behavior, relaxation, and sleep. GABA is endogenously produced in the brain from the amino acid glutamate and is responsible for inhibiting the activity of nerve cells in the brain and central nervous system. GABA is a popular dietary supplement and is used to help reduce anxiety and improve sleep, but more research is needed to determine its safety and effectiveness.
See More Information Regarding Gamma-aminobutyric Acid (gaba)| Ingredient Group | GABA |
|---|---|
| Category | non-nutrient/non-botanical |
Hops
Hops are the female flowers (also known as cones) of the hop plant, Humulus lupulus, and a member of the Cannabaceae family. Hops are native to Europe and are widely cultivated in many parts of the world. Hops have a variety of applications, such as for food flavoring and brewing beer. Hops extract has been used in topical medicinal preparations and as a dietary supplement.
See More Information Regarding Hops| Ingredient Group | Hops |
|---|---|
| Category | botanical |
Lavender
Lavender is a perennial herb native to the Mediterranean region that has been used for centuries for its aroma in commercial products, as well as in herbal medicine and aromatherapy. It has a sweet, floral aroma and is often used to promote relaxation and relieve stress. In traditional medicine, lavender has been mainly used for its purported anxiolytic (anxiety-reducing) effects. Lavender essential oil has also been purported to have antibacterial, antifungal, and antiviral properties and may be helpful in the treatment of certain skin conditions, such as acne and dandruff.
See More Information Regarding Lavender| Ingredient Group | English Lavender |
|---|---|
| Category | botanical |
Corydalis
| Ingredient Group | Corydalis |
|---|---|
| Category | botanical |
Passion Flower
Passionflower (Passiflora incarnata) is a climbing vine with fragrant white and purple flowers that is native to the Americas. It is purported to have a number of potential health benefits, including reducing anxiety and improving sleep. It is thought to work by increasing the levels of GABA (gamma-aminobutyric acid), a neurotransmitter in the brain that helps regulate mood and anxiety. Passionflower may also have mild sedative and muscle-relaxing effects and may be helpful in the treatment of muscle spasms and other muscle disorders. Dietary supplements containing passionflower are generally standardized to contain no less than 1.5% of the flavanoid vitexin.
See More Information Regarding Passion Flower| Ingredient Group | Passionflower |
|---|---|
| Category | botanical |
Melatonin
Melatonin is a hormone that is produced naturally in the pineal gland. It is involved in several different bodily processes, such as the regulation of the body's sleep-wake cycle. Melatonin is available as a dietary supplement and is often used to help people with sleep disorders, such as insomnia, fall asleep more easily. There is some evidence to suggest that melatonin supplements may be helpful in reducing the time it takes to fall asleep and improving sleep quality, although the results of studies on this topic have been mixed. Studies show better support for some specific conditions, such as Delayed sleep phase syndrome (DSPS) and Non-24-hour sleep-wake disorder. Melatonin is generally considered to be safe when used in the short term, although it can cause side effects in some people, such as dizziness, headache, and nausea. While melatonin is a natural hormone, it is often synthesized for its use in dietary supplements.
See More Information Regarding Melatonin| Ingredient Group | Melatonin |
|---|---|
| Category | hormone |
Drugs that interact with Ambitropin 1200 mg by Cellusyn Laboratories
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
Label Information
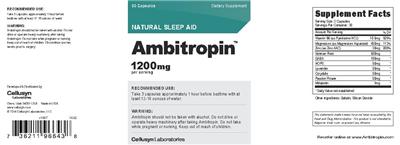
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
3 Capsule(s)
|
| Maximum serving Sizes: |
3 Capsule(s)
|
| Servings per container | 30 |
| UPC/BARCODE | 736211966438 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Vitamin B6 |
10.5 mg
|
Vitamin B6 |
525%
|
| Magnesium |
450 mg
|
Magnesium |
113%
|
| Zinc |
30 mg
|
Zinc |
200%
|
| Valerian |
500 mg
|
Valerian |
--
|
| GABA |
100 mg
|
GABA |
--
|
| Hops |
50 mg
|
Hops |
--
|
| Lavender |
50 mg
|
English Lavender |
--
|
| Corydalis |
50 mg
|
Corydalis |
--
|
| Passion Flower |
50 mg
|
Passionflower |
--
|
| Melatonin |
3 mg
|
Melatonin |
--
|
| Other Ingredients: |
Gelatin
Silicon Dioxide
|
|---|
Label Statments:
| Suggested/Recommended/Usage/Directions |
- RECOMMENDED USE: Take 3 capsules approximately 1 hour before bedtime with at least 12-16 ounces of water.
|
|---|---|
| Precautions |
- WARNING:
Ambitropin should not be taken with alcohol. Do not drive or operate heavy machinery after taking Ambitropin.
- Do not take while pregnant or nursing.
- Keep out of reach of children.
- Discontinue use two weeks prior to surgery.
|
| Brand IP Statement(s) |
- (c) 2014 Cellusyn Laboratories, LLC
|
| General |
- v1007 14.02
|
| FDA Statement of Identity |
- Dietary Supplement
|
| General Statements |
- NATURAL SLEEP AID
- 1200mg per serving
- Reorder online at www.Ambitropin.com
- Made in USA
|
| FDA Disclaimer Statement |
- The statements made hereon have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease
|
Brand Information
See all products by this brand
| Developed & Distributed by | |
|---|---|
| Name | Cellusyn Laboratories |
| City | Orem |
| State | Utah |
| Country | USA |
| ZipCode | 84057 |
| Web Address | www.cellusyn.com |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.