Glucosamine HCl 1500 mg By Safeway Overview & Drug Interactions
Check For Interactions With Glucosamine HCl 1500 mg
Supplement: Glucosamine HCl 1500 mg by Safeway
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Glucosamine Hydrochloride
Glucosamine is a natural compound found in the body, particularly in the fluid that surrounds joints. It is often used as a dietary supplement to help with joint health, particularly for conditions such as osteoarthritis. Glucosamine is believed to work by helping to reduce inflammation, promoting the repair of damaged cartilage, and slowing down the loss of cartilage. There are several different forms of glucosamine supplements, including glucosamine sulfate, glucosamine hydrochloride, and N-acetyl-glucosamine. Glucosamine sulfate is the most commonly used form in supplements. It is important to note that glucosamine sulfate is derived from shellfish (specifically the exoskeletons of shrimp, lobster, and crabs) and can be problematic for people with shellfish allergies.
See More Information Regarding Glucosamine| Ingredient Group | Glucosamine Hydrochloride |
|---|---|
| Category | non-nutrient/non-botanical |
Drugs that interact with Glucosamine HCl 1500 mg by Safeway
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
Label Information
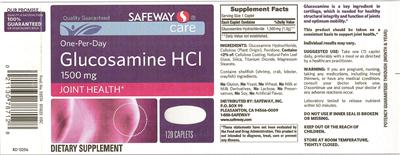
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
1 Caplet(s)
|
| Maximum serving Sizes: |
1 Caplet(s)
|
| UPC/BARCODE | 021130787128 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Glucosamine Hydrochloride |
1500 mg
|
Glucosamine Hydrochloride |
--
|
| Other Ingredients: |
Glucosamine Hydrochloride
Cellulose
Povidone
Contains <2% of:
Forms
|
|---|
Label Statments:
| Seals/Symbols |
- Our promise
Quality satisfaction 100% guaranteed or your money back
- Prod. No. 53574 B11822 05C RD 13256
|
|---|---|
| General Statements |
- Quality guaranteed
- Care
- Joint health
- Glucosamine is a key ingredient in cartilage, which is needed for healthy structural integrity and function of joints and optimum mobility.
- Individual results may vary.
|
| Suggested/Recommended/Usage/Directions |
- One-per-day
- This product should be taken on consistent basis to support joint health.
- Suggested Use: Take one (1) caplet daily, preferably with a meal or as directed by a healthcare practitioner.
|
| FDA Statement of Identity |
- Dietary Supplement
|
| Formula |
- Contains shellfish (shrimp, crab, lobster, crayfish) ingredients.
|
| Precautions |
- Contains shellfish (shrimp, crab, lobster, crayfish) ingredients.
- Warning: If you are pregnant, nursing, taking any medications, including blood thinners, or have any medical condition, consult your doctor before use.
- Discontinue use and consult your doctor if any adverse reactions occur.
Do not use if inner seal is broken or missing.
- Keep out of the reach of children.
|
| Formulation |
- No gluten, no yeast, no wheat, no milk or milk derivatives, no lactose, no preservatives, no soy, no artificial flavor.
|
| FDA Disclaimer Statement |
- These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
|
| Storage |
- Store at room temperature, tightly closed.
|
Brand Information
See all products by this brand
| Distributed by: | |
|---|---|
| Name | SAFEWAY, INC. |
| Street Address | P.O. BOX 99 |
| City | PLEASANTON |
| State | CA |
| ZipCode | 94566-0009 |
| Phone Number | 1 888-723-3929 |
| Web Address | www.safeway.com |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.