SynovoDerma By Allergy Research Group Overview & Drug Interactions
Check For Interactions With SynovoDerma
Supplement: SynovoDerma by Allergy Research Group
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Hyaluronic Acid powder
| Ingredient Group | Hyaluronic acid |
|---|---|
| Category | non-nutrient/non-botanical |
- Hyaluronic Acid (9%)
Drugs that interact with SynovoDerma by Allergy Research Group
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
There were no interactions found with SynovoDerma. This does not mean the potential for an interaction does not exist, however. There is often a lack of studies and data surrounding traditional medicine, especially concerning drug interactions, so it is important to always consult your provider before making any changes to your medication regimen.
Label Information
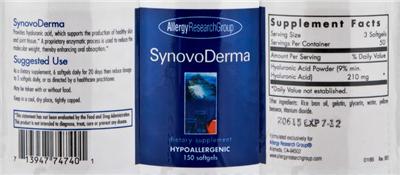
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
3 Softgel(s)
|
| Maximum serving Sizes: |
6 Softgel(s)
|
| Servings per container | 50 |
| UPC/BARCODE | 713947747401 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Hyaluronic Acid powder |
210 mg
|
Hyaluronic acid |
--
|
| Other Ingredients: |
Rice Bran Oil
Gelatin
Glycerin
Water
yellow Beeswax
Titanium Dioxide
|
|---|
Label Statments:
| General Statements |
- 20615EXP 7-12
- Provides hyaluronic acid, which supports the production of healthy skin and joint tissue.*
- A proprietary enzymatic process is used to reduce the molecular weight, thereby enhancing oral absorption.*
|
|---|---|
| Suggested/Recommended/Usage/Directions |
- Suggested Use
As a dietary supplement, 6 softgels daily for 20 days then reduce dosage to 3 softgels daily, or as directed by a healthcare practitioner.
May be taken with or without food.
|
| Storage |
- Keep in a cool, dry place, tightly capped.
|
| FDA Disclaimer Statement |
- This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
|
| FDA Statement of Identity |
- dietary supplement
|
| Formula |
- HYPOALLERGENIC
|
| General |
- Rev. 001
|
Brand Information
See all products by this brand
| Formulated exclusively for | |
|---|---|
| Name | Allergy Research Group |
| City | Alameda |
| State | CA |
| ZipCode | 94502 |
| Web Address | www.allergyresearchgroup.com |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.