Search by Drug Name or NDC
Search: Prednisolone
| # | NDC | Generic Drug Name | Drug Name | HIC3 Description |
|---|---|---|---|---|
| 1 | 00121075908 | Prednisolone Sod Phosphate Oral Soln 15 MG/5ML (Base Equiv) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 2 | 00121077308 | Prednisolone Sod Phosphate Oral Soln 10 MG/5ML (Base Equiv) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 3 | 00121077708 | Prednisolone Sod Phosphate Oral Soln 20 MG/5ML (Base Equiv) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 4 | 00121090204 | Prednisolone Sod Phosph Oral Soln 6.7 MG/5ML (5 MG/5ML Base) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 5 | 13925016604 | Prednisolone Sod Phosph Oral Soln 6.7 MG/5ML (5 MG/5ML Base) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 6 | 24208031705 | Sulfacetamide Sodium-Prednisolone Ophth Soln 10-0.23(0.25)% | Sulfacetamide-prednisoLONE | EYE SULFONAMIDES |
| 7 | 24208071510 | Prednisolone Sodium Phosphate Ophth Soln 1% | prednisoLONE Sodium Phosphate | EYE ANTI-INFLAMMATORY AGENTS |
| 8 | 42799081201 | Prednisolone Sod Phosphate Oral Soln 10 MG/5ML (Base Equiv) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 9 | 42799081301 | Prednisolone Sod Phosphate Oral Soln 20 MG/5ML (Base Equiv) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 10 | 44523018208 | Prednisolone Sodium Phosphate Oral Soln 25 MG/5ML (Base Eq) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 11 | 50090055900 | Prednisolone Acetate Ophth Susp 1% | prednisoLONE Acetate | EYE ANTI-INFLAMMATORY AGENTS |
| 12 | 50090065500 | Prednisolone Syrup 15 MG/5ML (USP Solution Equivalent) | prednisoLONE | GLUCOCORTICOIDS |
| 13 | 50090065501 | Prednisolone Syrup 15 MG/5ML (USP Solution Equivalent) | prednisoLONE | GLUCOCORTICOIDS |
| 14 | 50090158200 | Prednisolone Sod Phosphate Oral Soln 15 MG/5ML (Base Equiv) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 15 | 50383004224 | Prednisolone Syrup 15 MG/5ML (USP Solution Equivalent) | prednisoLONE | GLUCOCORTICOIDS |
| 16 | 50383004248 | Prednisolone Syrup 15 MG/5ML (USP Solution Equivalent) | prednisoLONE | GLUCOCORTICOIDS |
| 17 | 60758011905 | Prednisolone Acetate Ophth Susp 1% | prednisoLONE Acetate | EYE ANTI-INFLAMMATORY AGENTS |
| 18 | 60758011910 | Prednisolone Acetate Ophth Susp 1% | prednisoLONE Acetate | EYE ANTI-INFLAMMATORY AGENTS |
| 19 | 60758011915 | Prednisolone Acetate Ophth Susp 1% | prednisoLONE Acetate | EYE ANTI-INFLAMMATORY AGENTS |
| 20 | 61314063705 | Prednisolone Acetate Ophth Susp 1% | prednisoLONE Acetate | EYE ANTI-INFLAMMATORY AGENTS |
| 21 | 61314063710 | Prednisolone Acetate Ophth Susp 1% | prednisoLONE Acetate | EYE ANTI-INFLAMMATORY AGENTS |
| 22 | 61314063715 | Prednisolone Acetate Ophth Susp 1% | prednisoLONE Acetate | EYE ANTI-INFLAMMATORY AGENTS |
| 23 | 66993084435 | Prednisolone Sod Phos Orally Disintegr Tab 10 MG (Base Eq) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 24 | 66993084535 | Prednisolone Sod Phos Orally Disintegr Tab 15 MG (Base Eq) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 25 | 66993084635 | Prednisolone Sod Phos Orally Disintegr Tab 30 MG (Base Eq) | prednisoLONE Sodium Phosphate | GLUCOCORTICOIDS |
| 26 | 68788770802 | Prednisolone Sod Phosphate Oral Soln 15 MG/5ML (Base Equiv) | prednisoLONE Sodium Phosphate | N/A |
| 27 | 11980017405 | Prednisolone Acetate Ophth Susp 0.12% | Pred Mild | EYE ANTI-INFLAMMATORY AGENTS |
| 28 | 11980017410 | Prednisolone Acetate Ophth Susp 0.12% | Pred Mild | EYE ANTI-INFLAMMATORY AGENTS |
| 29 | 11980018001 | Prednisolone Acetate Ophth Susp 1% | Pred Forte | EYE ANTI-INFLAMMATORY AGENTS |
| 30 | 11980018005 | Prednisolone Acetate Ophth Susp 1% | Pred Forte | EYE ANTI-INFLAMMATORY AGENTS |
What Is This Tool?
This tool allows you to look up the NDC (National Drug Code) and associated information of any commercial drug by utilizing a variety of search terms.
All NDCs of a given drug in the search results are hyperlinks that direct to pages that provide detailed NDC and drug information, including:
- Drug Name
- Drug Strength
- NDC
- Active Ingredient
- Package images
- Full Prescribing Information (Structured Product Labeling)
- Proprietary Names
- GCN (Generic Code Number)
- GCN Sequence Number (also known as GSN)
- HICL Sequence Number
- HIC3 (Hierarchical Ingredient Code 3)
- Pricing
- And more...
This tool is free to use but its contents are not available for download or reproduction.
Example Searches
- NDC to Drug: Type in the NDC '31722021490' to bring up results for the associated drug (sertraline 100mg tablets)
- Drug to NDC: Type in the drug 'pravastatin 10mg' to bring up results for the associated NDCs
- NDC Lookup: Type in the NDC to display the associated drug name. The NDC codes are hyperlinks to further detail.
What Is NDC?
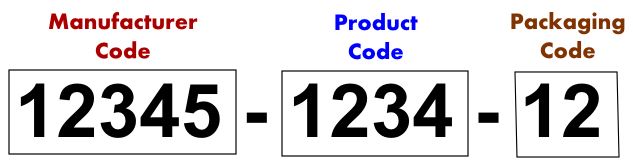
NDC stands for National Drug and is a unique product identifier used in the United States. It consists of 3 groupings of numbers that identify:
- Manufacturer
- Product (i.e. drug, strength, and dosage form)
- Package (i.e. package size quantity)