Search by Drug Name or NDC
NDC 00009-7667-05 Cleocin 100 mg/1 Details
Cleocin 100 mg/1
Cleocin is a VAGINAL SUPPOSITORY in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Pharmacia & Upjohn Company LLC. The primary component is CLINDAMYCIN PHOSPHATE.
Product Information
| NDC | 00009-7667 |
|---|---|
| Product ID | 0009-7667_2f2c2547-3d8d-4904-b155-23eff23b7ce9 |
| Associated GPIs | 55100018105220 |
| GCN Sequence Number | 044397 |
| GCN Sequence Number Description | clindamycin phosphate SUPP.VAG 100 MG VAGINAL |
| HIC3 | Q4W |
| HIC3 Description | VAGINAL ANTIBIOTICS |
| GCN | 91969 |
| HICL Sequence Number | 004045 |
| HICL Sequence Number Description | CLINDAMYCIN PHOSPHATE |
| Brand/Generic | Brand |
| Proprietary Name | Cleocin |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | clindamycin phosphate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SUPPOSITORY |
| Route | VAGINAL |
| Active Ingredient Strength | 100 |
| Active Ingredient Units | mg/1 |
| Substance Name | CLINDAMYCIN PHOSPHATE |
| Labeler Name | Pharmacia & Upjohn Company LLC |
| Pharmaceutical Class | Decreased Sebaceous Gland Activity [PE], Lincosamide Antibacterial [EPC], Lincosamides [CS], Neuromuscular Blockade [PE] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA050767 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images



NDC 00009-7667-05 (00009766705)
| NDC Package Code | 0009-7667-05 |
|---|---|
| Billing NDC | 00009766705 |
| Package | 1 BLISTER PACK in 1 CARTON (0009-7667-05) / 3 SUPPOSITORY in 1 BLISTER PACK |
| Marketing Start Date | 2022-11-18 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 3db26227-208d-4fdd-8426-1d9be5cda9b4 Details
DESCRIPTION
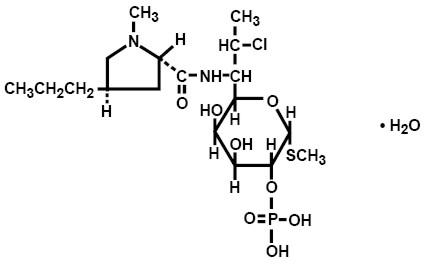
Clindamycin phosphate is a water-soluble ester of the semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin. The chemical name for clindamycin phosphate is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate). The monohydrate form has a molecular weight of 522.98, and the molecular formula is C18H34ClN2O8PS•H2O. The structural formula is represented below:
CLEOCIN Vaginal Ovules are semisolid, white to off-white suppositories for intravaginal administration. Each 2.5 g suppository contains clindamycin phosphate equivalent to 100 mg clindamycin in a base consisting of a mixture of glycerides of saturated fatty acids.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Systemic absorption of clindamycin was estimated following a once-a-day intravaginal dose of one clindamycin phosphate vaginal suppository (equivalent to 100 mg clindamycin) administered to 11 healthy female volunteers for 3 days. Approximately 30% (range 6% to 70%) of the administered dose was absorbed systemically on day 3 of dosing based on area under the concentration-time curve (AUC). Systemic absorption was estimated using a subtherapeutic 100 mg intravenous dose of clindamycin phosphate as a comparator in the same volunteers. The mean AUC following day 3 of dosing with the suppository was 3.2 µg hr/mL (range 0.42 to 11 µg hr/mL). The Cmax observed on day 3 of dosing with the suppository averaged 0.27 µg/mL (range 0.03 to 0.67 µg/mL) and was observed about 5 hours after dosing (range 1 to 10 hours). In contrast, the AUC and Cmax after the single intravenous dose averaged 11 µg hr/mL (range 5.1 to 26 µg hr/mL) and 3.7 µg/mL (range 2.4 to 5.0 µg/mL), respectively. The mean apparent elimination half-life after dosing with the suppository was 11 hours (range 4 to 35 hours) and is considered to be limited by the absorption rate.
The results from this study showed that systemic exposure to clindamycin (based on AUC) from the suppository was, on average, three-fold lower than that from a single subtherapeutic 100 mg intravenous dose of clindamycin. In addition, the recommended daily and total doses of intravaginal clindamycin suppository are far lower than those typically administered in oral or parenteral clindamycin therapy (100 mg of clindamycin per day for 3 days equivalent to about 30 mg absorbed per day from the ovule relative to 600 to 2700 mg/day for up to 10 days or more, orally or parenterally). The overall systemic exposure to clindamycin from Cleocin Vaginal Ovules is substantially lower than the systemic exposure from therapeutic doses of oral clindamycin hydrochloride (two-fold to 20-fold lower) or parenteral clindamycin phosphate (40-fold to 50-fold lower).
MICROBIOLOGY
Mechanism of Action
Clindamycin inhibits bacterial protein synthesis by binding to the 23S RNA of the 50S subunit of the ribosome. Clindamycin is predominantly bacteriostatic.
Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts it to active clindamycin.
Resistance
Resistance to clindamycin is most often caused by modification of the target site on the ribosome, usually by chemical modification of RNA bases or by point mutations in RNA or occasionally in proteins. Cross resistance has been demonstrated between lincosamides, macrolides and streptogramins B in some organisms. Cross resistance has been demonstrated between clindamycin and lincomycin.
Antibacterial Activity
Culture and sensitivity testing of bacteria are not routinely performed to establish the diagnosis of bacterial vaginosis (see INDICATIONS AND USAGE); standard methodology for the susceptibility testing of the potential bacterial pathogens, Gardnerella vaginalis, Mobiluncus spp., or Mycoplasma hominis, has not been defined.
The following in vitro data are available but their clinical significance is unknown. Clindamycin is active in vitro against most isolates of the following organisms reported to be associated with bacterial vaginosis:
- Bacteroides spp.
- Gardnerella vaginalis
- Mobiluncus spp.
- Mycoplasma hominis
- Peptostreptococcus spp.
INDICATIONS AND USAGE
CLEOCIN Vaginal Ovules are indicated for 3-day treatment of bacterial vaginosis in non-pregnant women. There are no adequate and well-controlled studies of CLEOCIN Vaginal Ovules in pregnant women.
NOTE: For purposes of this indication, a clinical diagnosis of bacterial vaginosis is usually defined by the presence of a homogeneous vaginal discharge that (a) has a pH of greater than 4.5, (b) emits a "fishy" amine odor when mixed with a 10% KOH solution, and (c) contains clue cells on microscopic examination. Gram's stain results consistent with a diagnosis of bacterial vaginosis include (a) markedly reduced or absent Lactobacillus morphology, (b) predominance of Gardnerella morphotype, and (c) absent or few white blood cells.
Other pathogens commonly associated with vulvovaginitis, e.g., Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, Candida albicans, and herpes simplex virus, should be ruled out.
CONTRAINDICATIONS
CLEOCIN Vaginal Ovules are contraindicated in individuals with a history of hypersensitivity to clindamycin, lincomycin, or any of the components of this vaginal suppository. CLEOCIN Vaginal Ovules are also contraindicated in individuals with a history of regional enteritis, ulcerative colitis, or a history of "antibiotic-associated" colitis.
WARNINGS
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including clindamycin, and may range in severity from mild to life-threatening. Orally and parenterally administered clindamycin has been associated with severe colitis, which may end fatally. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of orally and parenterally administered clindamycin, as well as with topical (dermal and vaginal) formulations of clindamycin. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of CLEOCIN Vaginal Ovules, because approximately 30% of the clindamycin dose is systemically absorbed from the vagina.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridioides difficile is a primary cause of "antibiotic-associated" colitis.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridioides difficile colitis.
Onset of pseudomembranous colitis symptoms may occur during or after antimicrobial treatment.
PRECAUTIONS
General
The use of CLEOCIN Vaginal Ovules may result in the overgrowth of nonsusceptible organisms in the vagina. In clinical studies using CLEOCIN Vaginal Ovules, treatment-related moniliasis was reported in 2.7% and vaginitis in 3.6% of 589 nonpregnant women. Moniliasis, as reported here, includes the terms: vaginal or nonvaginal moniliasis and fungal infection. Vaginitis includes the terms: vulvovaginal disorder, vaginal discharge, and vaginitis/vaginal infection.
Information for the Patient
The patient should be instructed not to engage in vaginal intercourse or use other vaginal products (such as tampons or douches) during treatment with this product.
The patient should also be advised that these suppositories use an oleaginous base that may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms. Therefore, the use of such products within 72 hours following treatment with CLEOCIN Vaginal Ovules is not recommended.
Drug Interactions
Systemic clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed included a rat micronucleus test and an Ames test. Both tests were negative. Fertility studies in rats treated orally with up to 300 mg/kg/day (31 times the human exposure based on mg/m2) revealed no effects on fertility or mating ability.
Pregnancy
Teratogenic effects
In clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters, has not been associated with an increased frequency of congenital abnormalities.
Clindamycin vaginal ovules should be used during the first trimester of pregnancy only if clearly needed and the benefits outweigh the risks. There are no adequate and well-controlled studies of CLEOCIN Vaginal Ovules in pregnant women during the first trimester of pregnancy.
CLEOCIN Vaginal Cream, 2%, has been studied in pregnant women during the second trimester. In women treated for 7 days, abnormal labor was reported more frequently in patients who received CLEOCIN Vaginal Cream compared to those receiving placebo (1.1% vs. 0.5% of patients, respectively).
Reproduction studies have been performed in rats and mice using oral and parenteral doses of clindamycin up to 600 mg/kg/day (62 and 25 times, respectively, the maximum human dose based on body surface area) and have revealed no evidence of harm to the fetus due to clindamycin. Cleft palates were observed in fetuses from one mouse strain treated intraperitoneally with clindamycin at 200 mg/kg/day (about 10 times the recommended dose based on body surface area conversions). Since this effect was not observed in other mouse strains or in other species, the effect may be strain specific.
Nursing Mothers
Limited published data based on breast milk sampling reports that clindamycin appears in human breast milk in the range of less than 0.5 to 3.8 mcg/mL at dosages of 150 mg orally to 600 mg intravenously. It is not known if clindamycin is excreted in human breast milk following the use of vaginally administered clindamycin phosphate.
Clindamycin has the potential to cause adverse effects on the breast-fed infant's gastrointestinal flora. If clindamycin is required by a nursing mother, it is not a reason to discontinue breastfeeding, but an alternate drug may be preferred. Monitor the breast-fed infant for possible adverse effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clindamycin and any potential adverse effects on the breast-fed child from clindamycin or from the underlying maternal condition.
Pediatric Use
The safety and efficacy of CLEOCIN Vaginal Ovules in the treatment of bacterial vaginosis in post-menarchal females have been established on the extrapolation of clinical trial data from adult women. When a post-menarchal adolescent presents to a health professional with bacterial vaginosis symptoms, a careful evaluation for sexually transmitted diseases and other risk factors for bacterial vaginosis should be considered. The safety and efficacy of CLEOCIN Vaginal Ovules in pre-menarchal females have not been established.
ADVERSE REACTIONS
Clinical Trials
In clinical trials, 3 (0.5%) of 589 nonpregnant women who received treatment with CLEOCIN Vaginal Ovules discontinued therapy due to drug-related adverse events. Adverse events judged to have a reasonable possibility of having been caused by clindamycin phosphate vaginal suppositories were reported for 10.5% of patients. Events reported by 1% or more of patients receiving CLEOCIN Vaginal Ovules were as follows:
Urogenital system: Vulvovaginal disorder (3.4%), vaginal pain (1.9%), and vaginal moniliasis (1.5%).
Body as a whole: Fungal infection (1.0%).
Other events reported by <1% of patients included:
Urogenital system: Menstrual disorder, dysuria, pyelonephritis, vaginal discharge, and vaginitis/vaginal infection.
Body as a whole: Abdominal cramps, localized abdominal pain, fever, flank pain, generalized pain, headache, localized edema, and moniliasis.
Digestive system: Diarrhea, nausea, and vomiting.
Skin: Nonapplication-site pruritis, rash, application-site pain, and application-site pruritis.
Other clindamycin formulations
The overall systemic exposure to clindamycin from CLEOCIN Vaginal Ovules is substantially lower than the systemic exposure from therapeutic doses of oral clindamycin hydrochloride (two-fold to 20-fold lower) or parenteral clindamycin phosphate (40-fold to 50-fold lower). (See CLINICAL PHARMACOLOGY.) Although these lower levels of exposure are less likely to produce the common reactions seen with oral or parenteral clindamycin, the possibility of these and other reactions cannot be excluded.
The following adverse reactions and altered laboratory tests have been reported with the oral or parenteral use of clindamycin and may also occur following administration of CLEOCIN Vaginal Ovules:
Infections and Infestations: Clostridioides difficile colitis
Gastrointestinal: Abdominal pain, esophagitis, nausea, vomiting, diarrhea, and pseudomembranous colitis. (See WARNINGS.)
Hematopoietic: Transient neutropenia (leukopenia), eosinophilia, agranulocytosis, and thrombocytopenia have been reported. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of these reports.
Hypersensitivity Reactions: Maculopapular rash and urticaria have been observed during drug therapy. Generalized mild to moderate morbilliform-like skin rashes are the most frequently reported of all adverse reactions. Cases of Acute Generalized Exanthematous Pustulosis (AGEP), erythema multiforme, some resembling Stevens-Johnson syndrome, have been associated with clindamycin. A few cases of anaphylactoid reactions have been reported. If a hypersensitivity reaction occurs, the drug should be discontinued.
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Musculoskeletal: Cases of polyarthritis have been reported.
Renal: Acute kidney injury
Immune System: Drug reaction with eosinophilia and systemic symptoms (DRESS) cases have been reported.
There have been reports of pseudomembranous colitis following the administration of clindamycin vaginal cream.
OVERDOSAGE
Vaginally applied clindamycin phosphate contained in CLEOCIN Vaginal Ovules could be absorbed in sufficient amounts to produce systemic effects. (See WARNINGS and ADVERSE REACTIONS.)
DOSAGE AND ADMINISTRATION
HOW SUPPLIED
SPL UNCLASSIFIED SECTION
Cleocin® Vaginal Ovules(clindamycin phosphate vaginal suppositories)
DIRECTIONS FOR USE
How do I use CLEOCIN Vaginal Ovules?
For vaginal use only. Do not take by mouth.
Use one CLEOCIN Vaginal Ovule daily, preferably at bedtime, for 3 days in a row.
Do not use this product if the foiled pouches containing vaginal ovules are torn, opened, or incompletely sealed.
Read the full directions below before using.
Insertion with the applicator:
- 1.
- Remove the vaginal ovule from its packaging (See Figure 1).
- 2.
- Pull back the plunger about an inch and place the vaginal ovule in the wider end of the applicator barrel (See Figure 2).

- 3.
- Hold the applicator as shown and gently insert the end of the applicator into the vagina as far as it will go comfortably. This can be done while lying on your back with your knees bent (as shown in Figure 3), or while standing with your feet apart and your knees bent.

- 4.
- While holding the barrel of the applicator in place, push the plunger in until it stops to release the vaginal ovule. Remove the applicator from the vagina.
- 5.
- Clean the applicator after each use. Pull the two pieces apart and wash them with soap and warm water. Rinse well and dry. Put the two pieces back together and store in a clean, dry place.
- 6.
- Once inside the vagina, the ovule melts. Lie down as soon as possible. This will keep leakage to a minimum.
- 7.
- Repeat steps 1 through 6, before bedtime, for the next 2 days.
Insertion without the applicator:
- Remove the vaginal ovule from its packaging (See Figure 1 above).
- Holding the ovule with your thumb and a finger, insert it into the vagina (See Figure 4). Figure 4
- Using your finger, gently push the ovule into the vagina as far as it will comfortably go (See Figure 5). Figure 5
- Once inside the vagina, the ovule melts. Lie down as soon as possible. This will keep leakage to a minimum.
- Repeat steps 1 through 4, before bedtime, for the next 2 days.
STORAGE CONDITIONS:
Store at 25°C (77°F); excursions permitted to 15 – 30°C (59 – 86°F) [see USP Controlled Room Temperature]. Caution: Avoid heat over 30°C (86°F). Avoid high humidity. See end of carton for the lot number and expiration date.

LAB-0649-2.0
Revised July 2022
PRINCIPAL DISPLAY PANEL - 100 mg Suppository Blister Pack
PRINCIPAL DISPLAY PANEL - 100 mg Suppository Blister Pack Carton
PRINCIPAL DISPLAY PANEL - 100 mg Suppository Blister Pack Carton - NDC 0009-7667-05
INGREDIENTS AND APPEARANCE
| CLEOCIN
clindamycin phosphate suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-7667) , MANUFACTURE(0009-7667) , API MANUFACTURE(0009-7667) , PACK(0009-7667) , LABEL(0009-7667) | |



