Search by Drug Name or NDC
NDC 00143-9006-01 AcetaZOLAMIDE 500 mg/1 Details
AcetaZOLAMIDE 500 mg/1
AcetaZOLAMIDE is a INTRAVENOUS INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Hikma Pharmaceuticals USA Inc.. The primary component is ACETAZOLAMIDE SODIUM.
Product Information
| NDC | 00143-9006 |
|---|---|
| Product ID | 0143-9006_6933faa5-ca70-4215-8f52-7055a688c287 |
| Associated GPIs | |
| GCN Sequence Number | 008163 |
| GCN Sequence Number Description | acetazolamide sodium VIAL 500 MG INJECTION |
| HIC3 | R1E |
| HIC3 Description | CARBONIC ANHYDRASE INHIBITORS |
| GCN | 34680 |
| HICL Sequence Number | 003640 |
| HICL Sequence Number Description | ACETAZOLAMIDE SODIUM |
| Brand/Generic | Generic |
| Proprietary Name | AcetaZOLAMIDE |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Acetazolamide |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 500 |
| Active Ingredient Units | mg/1 |
| Substance Name | ACETAZOLAMIDE SODIUM |
| Labeler Name | Hikma Pharmaceuticals USA Inc. |
| Pharmaceutical Class | Carbonic Anhydrase Inhibitor [EPC], Carbonic Anhydrase Inhibitors [MoA], Sulfonamides [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA040089 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

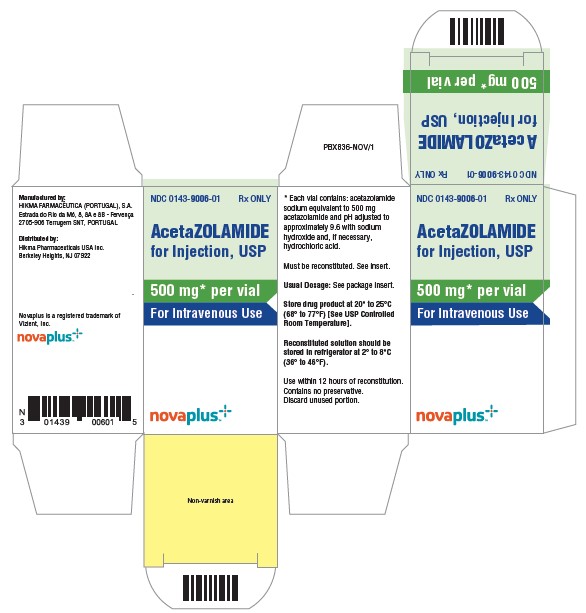

NDC 00143-9006-01 (00143900601)
| NDC Package Code | 0143-9006-01 |
|---|---|
| Billing NDC | 00143900601 |
| Package | 1 VIAL in 1 CARTON (0143-9006-01) / 1 INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION in 1 VIAL |
| Marketing Start Date | 1996-05-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 5502e5ba-9b5e-460a-855f-60534b625461 Details
DESCRIPTION
Acetazolamide, an inhibitor of the enzyme carbonic anhydrase, is a white to faintly yellowish white crystalline, odorless powder, weakly acidic, very slightly soluble in water and slightly soluble in alcohol. The chemical name for acetazolamide is N-(5-Sulfamoyl-1,3,4-thiadiazol-2yl)-acetamide and has the following structural formula:

M.W. 222.24 C4H6N4O3S2
Acetazolamide for Injection, USP is available for intravenous use, and is supplied as a sterile powder requiring reconstitution. Each vial contains acetazolamide sodium equivalent to 500 mg of acetazolamide. The bulk solution is adjusted to pH 9.6 using sodium hydroxide and, if necessary, hydrochloric acid prior to lyophilization.
CLINICAL PHARMACOLOGY
Acetazolamide is a potent carbonic anhydrase inhibitor, effective in the control of fluid secretion (e.g., some types of glaucoma), in the treatment of certain convulsive disorders (e.g., epilepsy) and in the promotion of diuresis in instances of abnormal fluid retention (e.g., cardiac edema).
Acetazolamide is not a mercurial diuretic. Rather, it is a nonbacteriostatic sulfonamide possessing a chemical structure and pharmacological activity distinctly different from the bacteriostatic sulfonamides.
Acetazolamide is an enzyme inhibitor that acts specifically on carbonic anhydrase, the enzyme that catalyzes the reversible reaction involving the hydration of carbon dioxide and the dehydration of carbonic acid. In the eye, this inhibitory action of acetazolamide decreases the secretion of aqueous humor and results in a drop in intraocular pressure, a reaction considered desirable in cases of glaucoma and even in certain nonglaucomatous conditions. Evidence seems to indicate that acetazolamide has utility as an adjuvant in the treatment of certain dysfunctions of the central nervous system (e.g., epilepsy). Inhibition of carbonic anhydrase in this area appears to retard abnormal, paroxysmal, excessive discharge from central nervous system neurons. The diuretic effect of acetazolamide is due to its action in the kidney on the reversible reaction involving hydration of carbon dioxide and dehydration of carbonic acid.
The result is renal loss of HCO3 ion, which carries out sodium, water, and potassium. Alkalinization of the urine and promotion of diuresis are thus effected. Alteration in ammonia metabolism occurs due to increased reabsorption of ammonia by the renal tubules as a result of urinary alkalinization.
INDICATIONS AND USAGE
For adjunctive treatment of: edema due to congestive heart failure; drug-induced edema; centrencephalic epilepsies (petit mal, unlocalized seizures); chronic simple (open-angle) glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where delay of surgery is desired in order to lower intraocular pressure.
CONTRAINDICATIONS
Hypersensitivity to acetazolamide or any excipients in the formulation. Since acetazolamide is a sulfonamide derivative, cross sensitivity between acetazolamide, sulfonamides and other sulfonamide derivatives is possible.
Acetazolamide therapy is contraindicated in situations in which sodium and/or potassium blood serum levels are depressed, in cases of marked kidney and liver disease or dysfunction, in suprarenal gland failure, and in hyperchloremic acidosis. It is contraindicated in patients with cirrhosis because of the risk of development of hepatic encephalopathy.
Long-term administration of acetazolamide is contraindicated in patients with chronic noncongestive angle-closure glaucoma since it may permit organic closure of the angle to occur while the worsening glaucoma is masked by lowered intraocular pressure.
WARNINGS
Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Sensitizations may recur when a sulfonamide is readministered irrespective of the route of administration. If signs of hypersensitivity or other serious reactions occur, discontinue use of this drug.
Caution is advised for patients receiving concomitant high-dose aspirin and acetazolamide, as anorexia, tachypnea, lethargy, coma and death have been reported.
PRECAUTIONS
General
Increasing the dose does not increase the diuresis and may increase the incidence of drowsiness and/or paresthesia. Increasing the dose often results in a decrease in diuresis. Under certain circumstances, however, very large doses have been given in conjunction with other diuretics in order to secure diuresis in complete refractory failure.
Information for Patients
Adverse reactions common to all sulfonamide derivatives may occur: anaphylaxis, fever, rash (including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), crystalluria, renal calculus, bone marrow depression, thrombocytopenic purpura, hemolytic anemia, leukopenia, pancytopenia and agranulocytosis. Precaution is advised for early detection of such reactions and the drug should be discontinued and appropriate therapy instituted.
In patients with pulmonary obstruction or emphysema where alveolar ventilation may be impaired, acetazolamide which may precipitate or aggravate acidosis, should be used with caution.
Caution is advised for patients receiving concomitant high-dose aspirin and acetazolamide, as anorexia, tachypnea, lethargy, coma and death have been reported (see WARNINGS).
Laboratory Tests
To monitor for hematologic reactions common to all sulfonamides, it is recommended that a baseline CBC and platelet count be obtained on patients prior to initiating acetazolamide therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. Periodic monitoring of serum electrolytes is recommended.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of acetazolamide have not been conducted. In a bacterial mutagenicity assay, acetazolamide was not mutagenic when evaluated with and without metabolic activation.
The drug had no effect on fertility when administered in the diet to male and female rats at a daily intake of up to 4 times the recommended human dose of 1000 mg in a 50 kg individual.
Pregnancy:
Teratogenic effects: Pregnancy Category C
Acetazolamide, administered orally or parenterally, has been shown to be teratogenic (defects of the limbs) in mice, rats, hamsters and rabbits. There are no adequate and well-controlled studies in pregnant women. Acetazolamide should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
ADVERSE REACTIONS
Adverse reactions, occurring most often early in therapy, include paresthesias, particularly a “tingling” feeling in the extremities, hearing dysfunction or tinnitus, loss of appetite, taste alteration and gastrointestinal disturbances such as nausea, vomiting and diarrhea; polyuria, and occasional instances of drowsiness and confusion.
Metabolic acidosis and electrolyte imbalance may occur.
Transient myopia has been reported. This condition invariably subsides upon diminution or discontinuance of the medication.
Other occasional adverse reactions include urticaria, melena, hematuria, glycosuria, hepatic insufficiency, flaccid paralysis, photosensitivity and convulsions. Also see PRECAUTIONS: Information for Patients for possible reactions common to sulfonamide derivatives. Fatalities have occurred although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia and other blood dyscrasias (see WARNINGS).
OVERDOSAGE
No data are available regarding acetazolamide overdosage in humans as no cases of acute poisoning with this drug have been reported. Animal data suggest that acetazolamide is remarkably nontoxic. No specific antidote is known. Treatment should be symptomatic and supportive.
Electrolyte imbalance, development of an acidotic state, and central nervous effects might be expected to occur. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored.
Supportive measures are required to restore electrolyte and pH balance. The acidotic state can usually be corrected by the administration of bicarbonate.
Despite its high intraerythrocytic distribution and plasma protein binding properties, acetazolamide may be dialyzable. This may be particularly important in the management of acetazolamide overdosage when complicated by the presence of renal failure.
DOSAGE AND ADMINISTRATION
Preparation and Storage of Parenteral Solution
Each 500 mg vial containing sterile acetazolamide sodium should be reconstituted with at least 5 mL of Sterile Water for Injection prior to use. Reconstituted solutions retain their physical and chemical properties for 3 days under refrigeration at 2° to 8°C (36° to 46°F), or 12 hours at room temperature 20° to 25°C (68° to 77°F). Contains no preservative. The direct intravenous route of administration is preferred. Intramuscular administration is not recommended.
Glaucoma
Acetazolamide should be used as an adjunct to the usual therapy. The dosage employed in the treatment of chronic simple (open-angle) glaucoma ranges from 250 mg to 1 g of acetazolamide per 24 hours, usually in divided doses for amounts over 250 mg. It has usually been found that a dosage in excess of 1 g per 24 hours does not produce an increased effect. In all cases, the dosage should be adjusted with careful individual attention both to symptomatology and ocular tension. Continuous supervision by a physician is advisable.
In treatment of secondary glaucoma and in the preoperative treatment of some cases of acute congestive (closed-angle) glaucoma, the preferred dosage is 250 mg every four hours, although some cases have responded to 250 mg twice daily on short-term therapy. In some acute cases, it may be more satisfactory to administer an initial dose of 500 mg followed by 125 or 250 mg every four hours depending on the individual case. Intravenous therapy may be used for rapid relief of ocular tension in acute cases. A complementary effect has been noted when acetazolamide has been used in conjunction with miotics or mydriatics as the case demanded.
Epilepsy
It is not clearly known whether the beneficial effects observed in epilepsy are due to direct inhibition of carbonic anhydrase in the central nervous system or whether they are due to the slight degree of acidosis produced by the divided dosage. The best results to date have been seen in petit mal in children. Good results, however, have been seen in patients, both children and adult, in other types of seizures such as grand mal, mixed seizure patterns, myoclonic jerk patterns, etc. The suggested total daily dose is 8 to 30 mg per kg in divided doses. Although some patients respond to a low dose, the optimum range appears to be from 375 to 1000 mg daily. However, some investigators feel that daily doses in excess of 1 g do not produce any better results than a 1 g dose. When acetazolamide is given in combination with other anticonvulsants, it is suggested that the starting dose should be 250 mg once daily in addition to the existing medications. This can be increased to levels as indicated above.
The change from other medications to acetazolamide should be gradual and in accordance with usual practice in epilepsy therapy.
Congestive Heart Failure
For diuresis in congestive heart failure, the starting dose is usually 250 to 375 mg once daily in the morning (5 mg per kg). If, after an initial response, the patient fails to continue to lose edema fluid, do not increase the dose but allow for kidney recovery by skipping medication for a day.
Acetazolamide yields best diuretic results when given on alternate days, or for two days alternating with a day of rest.
Failures in therapy may be due to overdosage or too frequent dosage. The use of acetazolamide does not eliminate the need for other therapy such as digitalis, bed rest, and salt restriction.
Drug-Induced Edema
Recommended dosage is 250 to 375 mg of acetazolamide once a day for one or two days, alternating with a day of rest.
Note: The dosage recommendations for glaucoma and epilepsy differ considerably from those for congestive heart failure, since the first two conditions are not dependent upon carbonic anhydrase inhibition in the kidney which requires intermittent dosage if it is to recover from inhibitory effect of the therapeutic agent.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
Acetazolamide for Injection, USP (lyophilized) powder.
NDC 0143-9006-01 500 mg Vial
Store drug product at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Reconstituted solution should be stored in refrigerator at 2° to 8°C (36° to 46°F). Use within 12 hours of reconstitution.
Contains no preservative. Discard unused portion.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
Manufactured by:
HIKMA FARMACÊUTICA (PORTUGAL), S.A.
Estrada do Rio da Mó, 8, 8A e 8B – Fervença – 2705-906 Terrugem SNT, PORTUGAL
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
Novaplus is a registered trademark of Vizient, Inc.
novaplus+
Revised April 2021
PIN575-NOV/1
Vial Principal Display Panel
Carton Principal Display Panel
INGREDIENTS AND APPEARANCE
| ACETAZOLAMIDE
acetazolamide injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hikma Pharmaceuticals USA Inc. (001230762) |
| Registrant - Hikma Pharmaceuticals USA Inc. (001230762) |