Search by Drug Name or NDC
NDC 00363-0428-12 Tension Headache Relief 500; 65 mg/1; mg/1 Details
Tension Headache Relief 500; 65 mg/1; mg/1
Tension Headache Relief is a ORAL TABLET, FILM COATED in the HUMAN OTC DRUG category. It is labeled and distributed by Walgreen Company. The primary component is ACETAMINOPHEN; CAFFEINE.
Product Information
| NDC | 00363-0428 |
|---|---|
| Product ID | 0363-0428_e3b2b757-6c17-43c3-ae3d-91d7f0337298 |
| Associated GPIs | 62100010002810 |
| GCN Sequence Number | 004930 |
| GCN Sequence Number Description | nicotine polacrilex GUM 2 MG BUCCAL |
| HIC3 | J3A |
| HIC3 Description | SMOKING DETERRENT AGENTS (GANGLIONIC STIM,OTHERS) |
| GCN | 03200 |
| HICL Sequence Number | 002049 |
| HICL Sequence Number Description | NICOTINE POLACRILEX |
| Brand/Generic | Generic |
| Proprietary Name | Tension Headache Relief |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Acetaminophen, Caffeine |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 500; 65 |
| Active Ingredient Units | mg/1; mg/1 |
| Substance Name | ACETAMINOPHEN; CAFFEINE |
| Labeler Name | Walgreen Company |
| Pharmaceutical Class | Central Nervous System Stimulant [EPC], Central Nervous System Stimulation [PE], Methylxanthine [EPC], Xanthines [CS] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Listing Certified Through | n/a |
Package
Package Images
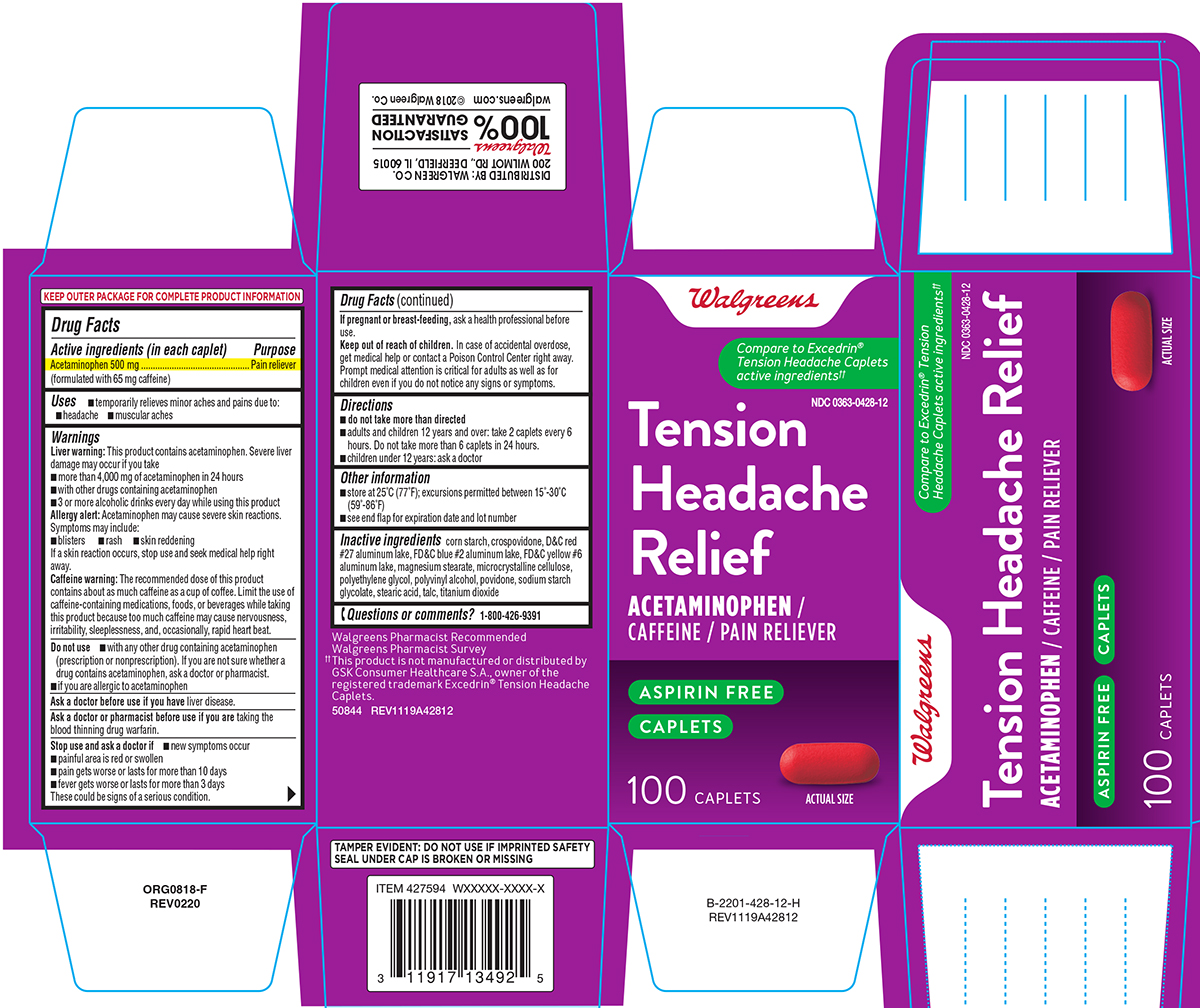
NDC 00363-0428-12 (00363042812)
| NDC Package Code | 0363-0428-12 |
|---|---|
| Billing NDC | 00363042812 |
| Package | 1 BOTTLE, PLASTIC in 1 CARTON (0363-0428-12) / 100 TABLET, FILM COATED in 1 BOTTLE, PLASTIC |
| Marketing Start Date | 2007-01-17 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 804b850c-962d-4d83-88d0-e0c21537e5f4 Details
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen
Directions
Other information
Inactive ingredients
Principal Display Panel
Walgreens
Compare to Excedrin® Tension
Headache Caplets active ingredients††
NDC 0363-0428-12
Tension Headache Relief
ACETAMINOPHEN / CAFFEINE / PAIN RELIEVER
ASPIRIN FREE
CAPLETS
100 CAPLETS
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSING
Walgreens Pharmacist Recommended
Walgreens Pharmacist Survey
††This product is not manufactured or distributed by
GSK group of companies, owner of the registered
trademark Excedrin® Tension Headache Caplets.
50844 REV1119A42812
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
Walgreens 100% SATISFACTION GUARANTEED
Walgreens.com ©2018 Walgreen Co.

44-428
INGREDIENTS AND APPEARANCE
| TENSION HEADACHE RELIEF
acetaminophen, caffeine tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Walgreen Company (008965063) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(0363-0428) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(0363-0428) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(0363-0428) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(0363-0428) | |