Search by Drug Name or NDC
NDC 00409-6476-44 Erythrocin Lactobionate 500 mg/100mL Details
Erythrocin Lactobionate 500 mg/100mL
Erythrocin Lactobionate is a INTRAVENOUS INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Hospira, Inc.. The primary component is ERYTHROMYCIN LACTOBIONATE.
Product Information
| NDC | 00409-6476 |
|---|---|
| Product ID | 0409-6476_ffa36ce2-7c68-4981-860c-f1538a2f9a63 |
| Associated GPIs | 03100050502105 |
| GCN Sequence Number | 059742 |
| GCN Sequence Number Description | erythromycin lactobionate VIAL PORT 500 MG INTRAVEN |
| HIC3 | W1D |
| HIC3 Description | MACROLIDE ANTIBIOTICS |
| GCN | 25529 |
| HICL Sequence Number | 004020 |
| HICL Sequence Number Description | ERYTHROMYCIN LACTOBIONATE |
| Brand/Generic | Brand |
| Proprietary Name | Erythrocin Lactobionate |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | ERYTHROMYCIN LACTOBIONATE |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 500 |
| Active Ingredient Units | mg/100mL |
| Substance Name | ERYTHROMYCIN LACTOBIONATE |
| Labeler Name | Hospira, Inc. |
| Pharmaceutical Class | Decreased Sebaceous Gland Activity [PE], Macrolide Antimicrobial [EPC], Macrolide [EPC], Macrolides [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA062638 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 00409-6476-44 (00409647644)
| NDC Package Code | 0409-6476-44 |
|---|---|
| Billing NDC | 00409647644 |
| Package | 10 VIAL, PATENT DELIVERY SYSTEM in 1 TRAY (0409-6476-44) / 100 mL in 1 VIAL, PATENT DELIVERY SYSTEM (0409-6476-54) |
| Marketing Start Date | 2006-03-16 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 9231d2fa-2e91-4eef-afa3-7515bc34df0b Details
SPL UNCLASSIFIED SECTION
Single-dose ADD-VantageTM Vials Rx only
For Intravenous Use Only

To reduce the development of drug-resistant bacteria and maintain the effectiveness of erythromycin and other antibacterial drugs, erythromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
ErythrocinTM Lactobionate-IV (erythromycin lactobionate for injection, USP) is a lyophilized powder for intravenous infusion only. It is prepared as a solution and lyophilized in its final container. The Erythrocin Lactobionate-IV ADD-VantageTM vial is designed for use only with the ADD-Vantage flexible diluent container. After appropriate dilution, the Erythrocin Lactobionate-IV ADD-Vantage Delivery System contains erythromycin lactobionate equivalent to either 500 mg of erythromycin activity in 100 mL.
The solutions contain no bacteriostat, antimicrobial agent (except erythromycin) or buffer and are intended for use as a single-dose injection only with the ADD-Vantage flexible diluent container.
Erythromycin is produced by a strain of Streptomyces erythraeus and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with acids. Erythromycin lactobionate has the following structure:
CLINICAL PHARMACOLOGY
Erythromycin diffuses readily into most body fluids. In the absence of meningeal inflammation, low concentrations are normally achieved in the spinal fluid but the passage of the drug across the blood-brain barrier increases in meningitis. Erythromycin crosses the placental barrier and is excreted in breast milk. Erythromycin is not removed by peritoneal dialysis or hemodialysis.
In the presence of normal hepatic function, erythromycin is concentrated in the liver and is excreted in the bile; the effect of hepatic dysfunction on biliary excretion of erythromycin is not known. From 12 to 15 percent of intravenously administered erythromycin is excreted in active form in the urine.
Intravenous infusion of 500 mg of erythromycin lactobionate at a constant rate over 1 hour in fasting adults produced a mean serum erythromycin level of approximately 7 mcg/mL at 20 minutes, 10 mcg/mL at 1 hour, 2.6 mcg/mL at 2.5 hours, and 1 mcg/mL at 6 hours.
Microbiology
Mechanism of Action
Erythromycin acts by inhibition of protein synthesis by binding 50 S ribosomal subunits of susceptible organisms. It does not affect nucleic acid synthesis.
Resistance
Resistance to erythromycin in S. aureus may emerge during therapy. Many isolates of Haemophilus influenzae are resistant to erythromycin but are susceptible to erythromycin and sulfonamides when used concomitantly.
Interactions with other Antimicrobials
Antagonism has been demonstrated in vitro between erythromycin and clindamycin, lincomycin and chloramphenicol.
Antimicrobial Activity
Aerobic bacteria
Gram-positive bacteria
- Corynebacterium diphtheriae
- Corynebacterium minutissimum
- Staphylococcus aureus
- Streptococcus pneumoniae
- Streptococcus pyogenes
Gram-negative bacteria
- Legionella pneumophila
- Neisseria gonorrhoeae
Other Microorganisms
- Mycoplasma pneumoniae
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for erythromycin against isolates of similar genus or organism group. However, the efficacy of erythromycin in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Aerobic bacteria
- Gram-negative bacteria
- Moraxella catarrhalis
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
INDICATIONS AND USAGE
Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the diseases listed below when oral administration is not possible or when the severity of the infection requires immediate high serum levels of erythromycin. Intravenous therapy should be replaced by oral administration at the appropriate time.
Upper respiratory tract infections of mild to moderate degree caused by Streptococcus pyogenes (Group A beta-hemolytic streptococci); Streptococcus pneumoniae (Diplococcus pneumoniae); Haemophilus influenzae (when used concomitantly with adequate doses of sulfonamides, since many strains of H. influenzae are not susceptible to the erythromycin concentrations ordinarily achieved). (See appropriate sulfonamide labeling for prescribing information)
Lower respiratory tract infections of mild to moderate severity caused by Streptococcus pyogenes (Group A beta-hemolytic streptococci); Streptococcus pneumoniae (Diplococcus pneumoniae).
Respiratory tract infections due to Mycoplasma pneumoniae.
Skin and skin structure infections of mild to moderate severity caused by Streptococcus pyogenes and Staphylococcus aureus (resistant staphylococci may emerge during treatment).
Diphtheria: As an adjunct to antitoxin infections due to Corynebacterium diphtheriae to prevent establishment of carriers and to eradicate the organism in carriers.
Erythrasma: In the treatment of infections due to Corynebacterium minutissimum.
Acute pelvic inflammatory disease caused by Neisseria gonorrhoeae: Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) followed by erythromycin stearate or erythromycin base orally, as an alternative drug in treatment of acute pelvic inflammatory disease caused by N. gonorrhoeae in female patients with a history of sensitivity to penicillin.
Before treatment of gonorrhea, patients who are suspected of also having syphilis should have a microscopic examination for T. pallidum (by immunofluorescence or darkfield) before receiving erythromycin and monthly serologic tests for a minimum of 4 months thereafter.
Legionnaires' Disease caused by Legionella pneumophila. Although no controlled clinical efficacy studies have been conducted, in vitro and limited preliminary clinical data suggest that erythromycin may be effective in treating Legionnaires' Disease.
Prevention of Initial Attacks of Rheumatic Fever
Penicillin is considered by the American Heart Association to be the drug of choice in the prevention of initial attacks of rheumatic fever (treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract e.g., tonsillitis, or pharyngitis).1 Erythromycin is indicated for the treatment of penicillin-allergic patients. The therapeutic dose should be administered for ten days.
Prevention of Recurrent Attacks of Rheumatic Fever
Penicillin or sulfonamides are considered by the American Heart Association to be the drugs of choice in the prevention of recurrent attacks of rheumatic fever. In patients who are allergic to penicillin and sulfonamides, oral erythromycin is recommended by the American Heart Association in the long-term prophylaxis of streptococcal pharyngitis (for the prevention of recurrent attacks of rheumatic fever).1
Prevention of Bacterial Endocarditis
Although no controlled clinical efficacy trials have been conducted, oral erythromycin has been recommended by the American Heart Association for prevention of bacterial endocarditis in penicillin-allergic patients with prosthetic cardiac valves, most congenital cardiac malformations, surgically constructed systemic pulmonary shunts, rheumatic or other acquired valvular dysfunction, idiopathic hypertrophic subaortic stenosis (IHSS), previous history of bacterial endocarditis and mitral valve prolapse with insufficiency when they undergo dental procedures and surgical procedures of the upper respiratory tract.2
To reduce the development of drug-resistant bacteria and maintain the effectiveness of erythromycin and other antibacterial drugs, erythromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
Erythromycin is contraindicated in patients with known hypersensitivity to this antibiotic. Erythromycin is contraindicated in patients taking terfenadine or astemizole, cisapride, pimozide, ergotamine, or dihydroergotamine (See WARNINGS and PRECAUTIONS – Drug Interactions).
Do not use erythromycin concomitantly with 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase inhibitors (statins) that are extensively metabolized by cytochrome P450 isoform 3A4 (lovastatin or simvastatin), due to the increased risk of myopathy, including rhabdomyolysis (See WARNINGS and PRECAUTIONS – Drug Interactions).
WARNINGS
Hepatotoxicity
There have been reports of hepatic dysfunction, with or without jaundice occurring in patients receiving oral erythromycin products. Since erythromycin is principally excreted by the liver, monitor for liver toxicity when erythromycin is administered to patients with impaired hepatic function (See CLINICAL PHARMACOLOGY).
Clostridioides difficile-Associated Diarrhea
- Clostridioides difficile-associated diarrhea (CDAD) has been reported with the use of nearly all antibacterial agents, including erythromycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
QT Prolongation
Life-threatening episodes of ventricular tachycardia associated with prolonged QT interval (torsades de pointes) have been reported in some patients after intravenous administration of erythromycin lactobionate.
Susceptibility to the development of torsades de pointes arrhythmias, a rare but serious cardiac condition, is related to electrolyte imbalance, hepatic dysfunction, myocardial ischemia, left ventricular dysfunction, idiopathic Q-T prolongation, and concurrent antiarrhythmic therapy.3 Elderly patients exhibit a greater frequency of decreased hepatic function, cardiac function, and of concomitant disease and other drug therapy, and therefore should be monitored carefully during ErythrocinTM therapy.
Infantile Hypertrophic Pyloric Stenosis (IHPS)
There have been reports of IHPS occurring in infants following erythromycin therapy. Since erythromycin may be used in the treatment of conditions in infants which are associated with significant mortality or morbidity (such as pertussis or chlamydia), the benefit of erythromycin therapy needs to be weighed against the potential risk of developing IHPS. Parents or caregivers of infants receiving erythromycin should be informed to contact their physician if vomiting or irritability with feeding occurs.
Drug Interactions
Serious adverse reactions have been reported in patients taking erythromycin concomitantly with CYP3A4 substrates. These include colchicine toxicity with colchicine; rhabdomyolysis with simvastatin, lovastatin, and atorvastatin; and hypotension with calcium channel blockers metabolized by CYP3A4 (e.g. verapamil, amlodipine, diltiazem, vasospasm and ischemia with ergotamine/dihydroergotamine) (See PRECAUTIONS – Drug Interactions).
PRECAUTIONS
Exacerbation of Myasthenia Gravis
There have been reports that erythromycin may aggravate the weakness of patients with myasthenia gravis.
Development of Drug-Resistant Bacteria
Prolonged or repeated use of erythromycin may result in an overgrowth of non-susceptible bacteria or fungi. If superinfection occurs, erythromycin should be discontinued and appropriate therapy instituted.
When indicated, incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy.
Prescribing erythromycin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Laboratory Tests
Erythromycin interferes with the fluorometric determination of urinary catecholamines.
Drug Interactions
Erythromycin administration in patients receiving 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase inhibitors (statins) that are extensively metabolized by cytochrome P450 isoform 3A4 (e.g., lovastatin or simvastatin) has been reported to cause increased risk of myopathy, including rhabdomyolysis. Do not administer erythromycin with lovastatin or simvastatin (See CONTRAINDICATIONS).
Erythromycin use in patients who are receiving high doses of theophylline may be associated with an increase of serum theophylline levels and potential theophylline toxicity. In case of theophylline toxicity and/or elevated serum theophylline levels, the dose of theophylline should be reduced while the patient is receiving concomitant erythromycin therapy.
There have been published reports suggesting that when oral erythromycin is given concurrently with theophylline there is a significant decrease in erythromycin serum concentrations. This decrease could result in subtherapeutic concentrations of erythromycin.
Erythromycin administration in patients receiving carbamazepine has been reported to cause increased serum levels of carbamazepine with subsequent development of signs of carbamazepine toxicity.
Concomitant administration of erythromycin and digoxin has been reported to result in elevated serum digoxin levels.
There have been reports of increased anticoagulant effects, which may be more pronounced in elderly when erythromycin and oral anticoagulants (e.g., warfarin) are used concomitantly.
Colchicine is a substrate for both CYP3A4 and the efflux transporter P-glycoprotein (P-gp). Erythromycin is considered a moderate inhibitor of CYP3A4. A significant increase in colchicine plasma concentration is anticipated when co-administered with moderate CYP3A4 inhibitors such as erythromycin. If co-administration of colchicine and erythromycin is necessary, the starting dose of colchicine may need to be reduced, and the maximum colchicine dose should be lowered. Patients should be monitored for clinical symptoms of colchicine toxicity (See WARNINGS).
Erythromycin has been reported to increase the systemic exposure (AUC) of sildenafil. Reduction of sildenafil dosage should be considered (See sildenafil prescribing information).
Erythromycin has been reported to decrease the clearance of triazolam, midazolam and related benzodiazepines, and thus may increase the pharmacological effect of these benzodiazepines.
Post-marketing reports indicate that co-administration of erythromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the central nervous system, extremities and other tissues (See CONTRAINDICATIONS).
Erythromycin has been reported to significantly alter the metabolism of the nonsedating antihistamines, terfenadine and astemizole, when taken concomitantly. Rare cases of serious cardiovascular adverse events, including electrocardiographic QT/QTc interval prolongation, cardiac arrest, torsades de pointes, and other ventricular arrhythmias, have been observed (See CONTRAINDICATIONS). In addition, deaths have been reported rarely with concomitant administration of terfenadine and erythromycin.
The use of erythromycin in patients concurrently taking drugs metabolized by the cytochrome P450 system may be associated with elevations in serum levels of these other drugs. There have been reports of interactions of erythromycin with carbamazepine, cyclosporine, hexobarbital, phenytoin, alfentanil, disopyramide, bromocriptine, valproate, terfenadine, and astemizole. Serum concentrations of drugs metabolized by the cytochrome P450 system should be monitored closely in patients concurrently receiving erythromycin.
Hypotension, bradyarrhythmias and lactic acidosis have been observed in patients receiving concurrent verapamil, a calcium-channel blocker.
Cimetidine may inhibit the metabolism of erythromycin, which may lead to an increased plasma concentration.
Erythromycin has been reported to decrease the clearance of racemic zopiclone and, thus, may also decrease the clearance of eszopiclone, the S-enantiomer of racemic zopiclone. Accordingly, erythromycin may increase the pharmacodynamic effects of eszopiclone. Dose reduction of eszopiclone may be necessary (See eszopiclone prescribing information).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal data with erythromycin lactobionate for use in determination of possible carcinogenic effects are not available. However, long-term oral studies in rats with erythromycin ethylsuccinate and erythromycin base did not provide evidence of tumorigenicity. Mutagenicity studies have not been conducted. There was no apparent effect on male or female fertility in rats fed erythromycin (base) at levels up to 0.25% of diet.
Pregnancy
There was no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base (up to 0.25% of diet) prior to and during mating, during gestation, and through weaning of two successive litters. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Erythromycin has been reported to cross the placental barrier in humans, but fetal plasma levels are generally low.
Labor and Delivery
The effect of erythromycin on labor and delivery is unknown.
Nursing Mothers
Erythromycin is excreted in breast milk. Caution should be exercised when erythromycin is administered to a nursing woman.
Pediatric Use
(See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION).
Geriatric Use
Elderly patients, particularly those with reduced renal or hepatic function, may be at increased risk for developing erythromycin-induced hearing loss, when ErythrocinTM doses of 4 grams/day or higher are given (See ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION).
Elderly patients may be more susceptible to the development of torsades de pointes arrhythmias than younger patients (See ADVERSE REACTIONS).
Elderly patients may experience increased effects of oral anticoagulant therapy while undergoing treatment with ErythrocinTM (See PRECAUTIONS - Drug Interactions).
Erythromycin Lactobionate does not contain sodium.
Information for Patients
Patients should be counseled that antibacterial drugs including erythromycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When erythromycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by erythromycin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
ADVERSE REACTIONS
Erythromycin has been associated with QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsades de pointes. (See WARNINGS).
Side effects following the use of intravenous erythromycin are rare. Occasional venous irritation has been encountered, but if the infusion is given slowly, in dilute solution, preferably by continuous intravenous infusion or intermittent infusion in no less than 20 to 60 minutes, pain and vessel trauma are minimized.
Allergic reactions ranging from urticaria to anaphylaxis have occurred. Skin reactions ranging from mild eruptions to erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported rarely.
There have been isolated reports of reversible hearing loss occurring chiefly in patients with renal insufficiency and in patients receiving high doses of erythromycin.
Elderly patients, particularly those with reduced renal or hepatic function, may also be at increased risk for developing this effect when ErythrocinTM doses of 4 grams/day or higher are given (See DOSAGE AND ADMINISTRATION).
OVERDOSAGE
In the case of overdosage, erythromycin infusion should be discontinued and all other appropriate measures should be instituted. Adverse reactions at higher than recommended doses could be similar to those reported with oral formulations of erythromycin, particularly, severe abdominal pain, nausea, vomiting, diarrhea, hepatitis, pancreatitis, and transient hearing loss.
Erythromycin is not removed by peritoneal dialysis or hemodialysis.
DOSAGE AND ADMINISTRATION
For the treatment of severe infections in adults and pediatric patients, the recommended intravenous dose of erythromycin lactobionate is 15 to 20 mg/kg/day. Higher doses, up to 4 g/day, may be given for severe infections.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function. Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) in the ADD-Vantage system must be administered by intermittent intravenous infusion only. Due to the irritative properties of erythromycin, IV push is an unacceptable route of administration.
Intravenous erythromycin should be replaced by oral erythromycin as soon as possible.
The drug should be administered as a single dose from the ADD-Vantage flexible diluent container. Discard any unused portion.
For intermittent infusion: administer one-fourth the total daily dose of erythromycin lactobionate by intravenous infusion in 20 to 60 minutes at intervals not greater than every six hours. The final diluted solution of erythromycin lactobionate is prepared to give a concentration of 1 to 5 mg/mL. No less than 100 mL of IV diluent should be used. Infusion should be sufficiently slow to minimize pain along the vein.
For treatment of acute pelvic inflammatory disease caused by N. Gonorrhoeae, in female patients hypersensitive to penicillins, administer 500 mg erythromycin lactobionate every six hours for three days, followed by oral administration of 250 mg erythromycin stearate or base every six hours for seven days.
For treatment of Legionnaires' Disease: Although optimal doses have not been established, doses utilized in reported clinical data were 1 to 4 grams daily in divided doses.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function.
In the treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract (e.g., tonsillitis or pharyngitis), the therapeutic dosage of erythromycin should be administered for ten days. The American Heart Association suggests a dosage of 250 mg of erythromycin orally, twice a day in long-term prophylaxis of streptococcal upper respiratory tract infections for the prevention of recurring attacks of rheumatic fever in patients allergic to penicillin and sulfonamides.1
In prophylaxis against bacterial endocarditis (See INDICATIONS AND USAGE) the oral regimen for penicillin allergic patients is erythromycin 1 gram, 1 hour before the procedure followed by 500 mg six hours later.2
Preparation of Solution:
The Erythrocin Lactobionate-IV ADD-Vantage vial may be used with either 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP in the ADD-Vantage flexible diluent container. The 500 mg ADD-Vantage vials must be used as single doses with the 100 mL ADD-Vantage flexible diluent containers. The resulting solution will contain erythromycin activity equal to approximately 5 mg/mL.
Do not administer unless solution is clear and container is undamaged. Discard any unused portion.
INSTRUCTIONS FOR USE
To Use Vial in ADD-Vantage Flexible Diluent Container
To Open:
Peel overwrap at corner and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
- •
- Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
- a.
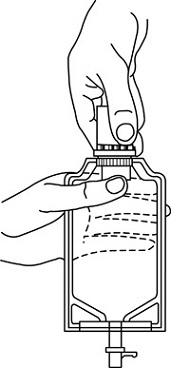
- To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (See FIGURE 1.), then pull straight up to remove the cap. (See FIGURE 2.) NOTE: Do not access vial with syringe.
Figure 1 Figure 2
-
- o
- To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (See FIGURE 3.)
- •
- Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (See FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go.
NOTE: Once vial is seated, do not attempt to remove. (See FIGURE 4.)
Figure 3 Figure 4
- •
- Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
- •
- Label appropriately.
To Reconstitute the Drug:
- •
- Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.
- •
- With the other hand, push the drug vial down into the container telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container. (See FIGURE 5.)
- •
- Pull the inner cap from the drug vial. (See FIGURE 6.) Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.
- •
- Mix container contents thoroughly and use within the specified time.
Figure 5 Figure 6
Preparation for Administration:
(Use Aseptic Technique)
- •
- Confirm the activation and admixture of vial contents.
- •
- Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
- •
- Close flow control clamp of administration set.
- •
- Remove cover from outlet port at bottom of container.
- •
- Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated.
NOTE: See full directions on administration set carton.
- •
- Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
- •
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- •
- Open flow control clamp and clear air from set. Close clamp.
- •
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- •
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible containers in series connections.
Stability:
In 0.9% Sodium Chloride Injection, USP
The final diluted solution of erythromycin lactobionate should be completely administered within 8 hours in order to assure proper potency.
In 5% Dextrose Injection, USP
The final diluted solution of erythromycin lactobionate should be completely administered within 2 hours in order to assure proper potency.
No drug or chemical agent should be added to an Erythrocin Lactobionate-IV fluid admixture unless its effect on the chemical and physical stability of the solution has first been determined.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
WARNING: Do not use flexible container in series connections.
HOW SUPPLIED
Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) is supplied as a sterile, lyophilized powder as follows:
|
NDC No. |
Container |
Concentration |
Quantity |
|
0409-6476-44 |
Single-dose ADD-Vantage Vial |
500 mg |
Tray of 10 |
Store at 20 to 25ºC (68 to 77ºF). [See USP Controlled Room Temperature].
REFERENCES
- •
- Committee on Rheumatic Fever and Infective Endocarditis of the Council on Cardiovascular Disease of the Young: Prevention of Rheumatic Fever, Circulation 70(6):1118A-1122A, December 1984.
- •
- Committee on Rheumatic Fever and Infective Endocarditis of the Council on Cardiovascular Disease of the Young: Prevention of Bacterial Endocarditis, Circulation 70(6):1123A-1127A, December 1984.
- •
- Gilter, B., et al, Torsades de Pointes Induced by Erythromycin, Chest, Volume 105: 368-72, February 1994.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA 
LAB-1219-4.0
Revised: 3/2020
PRINCIPAL DISPLAY PANEL - 500 mg Vial Label
Single-dose ADD-Vantage™ Vial
ERYTHROCIN™
Lactobionate - IV
Erythromycin Lactobionate for Injection, USP
Erythromycin Activity 500 mg per Vial
For IV Use Only
Sterile Powder for Injection
Preservative-Free
This single-dose vial is to be used only with the ADD-Vantage™ Flexible Diluent Container. When mixed as directed
with 100 mL of diluent, each 100 mL contains erythromycin lactobionate equivalent to 500 mg erythromycin.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
PRINCIPAL DISPLAY PANEL - 500 mg Vial Tray Label
INGREDIENTS AND APPEARANCE
| ERYTHROCIN LACTOBIONATE
erythromycin lactobionate injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 030606222 | ANALYSIS(0409-6476) , LABEL(0409-6476) , MANUFACTURE(0409-6476) , PACK(0409-6476) | |