Search by Drug Name or NDC
NDC 00409-7391-72 Sodium Phosphates 142; 276 mg/mL; mg/mL Details
Sodium Phosphates 142; 276 mg/mL; mg/mL
Sodium Phosphates is a INTRAVENOUS INJECTION, SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Hospira, Inc.. The primary component is SODIUM PHOSPHATE, DIBASIC, ANHYDROUS; SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE.
Product Information
| NDC | 00409-7391 |
|---|---|
| Product ID | 0409-7391_6c1b8eb9-c01e-4f9f-b12b-ccf64b5f5da1 |
| Associated GPIs | 79600020102030 |
| GCN Sequence Number | 001233 |
| GCN Sequence Number Description | sod phosphate,monobasic-dibas VIAL 3MMOL/ML INTRAVEN |
| HIC3 | C1P |
| HIC3 Description | PHOSPHATE REPLACEMENT |
| GCN | 03170 |
| HICL Sequence Number | 000540 |
| HICL Sequence Number Description | SODIUM PHOSPHATE,MONOBASIC-DIBASIC |
| Brand/Generic | Generic |
| Proprietary Name | Sodium Phosphates |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE and SODIUM PHOSPHATE, DIBASIC, ANHYDROUS |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, SOLUTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 142; 276 |
| Active Ingredient Units | mg/mL; mg/mL |
| Substance Name | SODIUM PHOSPHATE, DIBASIC, ANHYDROUS; SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE |
| Labeler Name | Hospira, Inc. |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA018892 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 00409-7391-72 (00409739172)
| NDC Package Code | 0409-7391-72 |
|---|---|
| Billing NDC | 00409739172 |
| Package | 25 VIAL, SINGLE-DOSE in 1 TRAY (0409-7391-72) / 15 mL in 1 VIAL, SINGLE-DOSE (0409-7391-82) |
| Marketing Start Date | 2005-06-02 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 6f044d16-295e-43ff-72a3-b045c387bc5c Details
SPL UNCLASSIFIED SECTION
DESCRIPTION
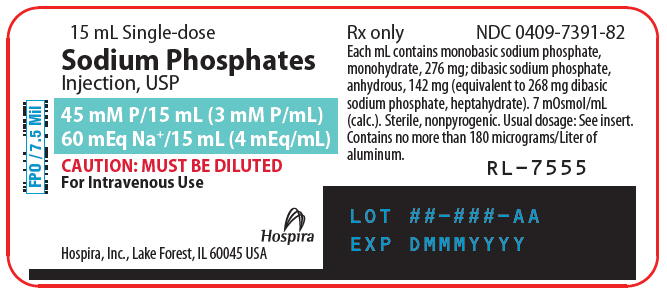
Sodium Phosphates Injection, USP, 3 mM P/mL (millimoles/mL), is a sterile, nonpyrogenic, concentrated solution containing a mixture of monobasic sodium phosphate and dibasic sodium phosphate in water for injection.
The solution is administered after dilution by the intravenous route as an electrolyte replenisher. It must not be administered undiluted.
Each mL contains 276 mg of monobasic sodium phosphate, monohydrate and 142 mg of dibasic sodium phosphate, anhydrous (equivalent to 268 mg of dibasic sodium phosphate, heptahydrate).
One mM of phosphorus weighs 31 mg, and the product provides 93 mg (approximately 3 mM) of phosphorus/mL plus 92 mg (4 mEq) of sodium/mL. Note: 1 mM P = 1 mM PO4. It contains no bacteriostat, antimicrobial agent or added buffer. The pH is 5.5 (5.0 to 6.0). The osmolar concentration is 7 mOsmol/mL (calc).
The solution is intended as an alternative to potassium phosphate to provide phosphorus for addition to large volume infusion fluids for intravenous use.
It is provided as a 15 mL partial fill single-dose vial; when lesser amounts are required, the unused portion should be discarded with the entire unit.
Monobasic Sodium Phosphate, USP (monohydrate) is chemically designated NaH2PO4• H2O, white, odorless crystals or granules freely soluble in water.
Dibasic Sodium Phosphate, USP (anhydrous) is chemically designated Na2HPO4, colorless or white granular salt freely soluble in water.
The semi-rigid container is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
CLINICAL PHARMACOLOGY
Phosphorus in the form of organic and inorganic phosphate has a variety of important biochemical functions in the body and is involved in many significant metabolic and enzyme reactions in almost all organs and tissues. It exerts a modifying influence on the steady state of calcium levels, a buffering effect on acid-base equilibrium and a primary role in the renal excretion of hydrogen ion.
Phosphorus is present in plasma and other extracellular fluid, in cell membranes and intracellular fluid, as well as in collagen and bone tissues. Phosphorus in the extracellular fluid is primarily in inorganic form and plasma levels may vary somewhat with age. The ratio of disodium phosphate and monosodium phosphate in the extracellular fluid is 4 to 1 (80% to 20%) at the normal pH of 7.4. This buffer ratio varies with the pH, but owing to its relatively low concentration, it contributes little to the buffering capacity of the extracellular fluid.
Phosphorus, present in large amounts in erythrocytes and other tissue cells, plays a significant intracellular role in the synthesis of high energy organic phosphates. It has been shown to be essential to maintain red cell glucose utilization, lactate production, and the concentration of both erythrocyte adenosine triphosphate (ATP) and 2,3 diphosphoglycerate (DPG), and must be deemed as important to other tissue cells. Hypophosphatemia should be avoided during periods of total parenteral nutrition, or other lengthy periods of intravenous infusions. It has been suggested that patients receiving total parenteral nutrition receive 12 to 15 mM phosphorus per 250 g of dextrose. Serum phosphorus levels should be regularly monitored and appropriate amounts of phosphorus should be added to the infusions to maintain normal serum phosphorus levels. Intravenous infusion of inorganic phosphorus may be accompanied by a decrease in the serum level and urinary excretion of calcium. The normal level of serum phosphorus is 3.0 to 4.5 mg/100 mL in adults; 4.0 to 7.0 mg/100 mL in children.
Intravenously infused phosphorus not taken up by the tissues is excreted almost entirely in the urine. Plasma phosphorus is believed to be filterable by the renal glomeruli, and the major portion of filtered phosphorus (greater than 80%) is actively reabsorbed by the tubules. Many modifying influences tend to alter the amount excreted in the urine.
Sodium is the principal cation of extracellular fluid. It comprises more than 90% of the total cations at its normal plasma concentration of approximately 142 mEq/liter. While the sodium ion can diffuse across cell membranes, intracellular sodium is maintained at a much lower concentration than extracellular sodium through the expenditure of energy by the cell (so called "sodium cation pump"). Loss of intracellular potassium ion is usually accompanied by an increase in intracellular sodium ion.
When serum sodium concentration is low, the secretion of antidiuretic hormone (ADH) by the pituitary is inhibited, thereby preventing water reabsorption by the distal renal tubules. On the other hand, adrenal secretion of aldosterone increases renal tubular reabsorption of sodium in an effort to re-establish normal serum sodium concentration.
INDICATIONS AND USAGE
Sodium Phosphates Injection, USP, 3 mM P/mL is indicated as a source of phosphorus, for addition to large volume intravenous fluids, to prevent or correct hypophosphatemia in patients with restricted or no oral intake. It is also useful as an additive for preparing specific parenteral fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions.
The concomitant amount of sodium (Na+ 4 mEq/mL) must be calculated into total electrolyte dose of such prepared solutions.
CONTRAINDICATIONS
WARNINGS
Sodium Phosphates Injection, USP, 3 mM P/mL must be diluted and thoroughly mixed before use.
To avoid phosphorus intoxication, infuse solutions containing sodium phosphate slowly. Infusing high concentrations of phosphorus may result in a reduction of serum calcium and symptoms of hypocalcemic tetany. Calcium levels should be monitored.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
PRECAUTIONS
Do not administer unless solution is clear and seal is intact. Discard unused portion.
Phosphorus replacement therapy with sodium phosphate should be guided primarily by the serum phosphorus level and the limits imposed by the accompanying sodium (Na+) ion.
Use with caution in patients with renal impairment, cirrhosis, cardiac failure and other edematous or sodium-retaining states.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Pregnancy: Animal reproduction studies have not been conducted with sodium phosphate. It is also not known whether sodium phosphate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium phosphate should be given to a pregnant woman only if clearly needed.
Pediatric Use: The safety and effectiveness of sodium phosphate has been established in pediatric patients (neonates, infants, children and adolescents).
Geriatric Use: An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Sodium ions and phosphorus are known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
ADVERSE REACTIONS
OVERDOSAGE
DOSAGE AND ADMINISTRATION
Sodium Phosphates Injection, USP, 3 mM P/mL is administered intravenously only after dilution and thorough mixing in a larger volume of fluid. The dose and rate of administration are dependent upon the individual needs of the patient. Serum sodium, phosphorus and calcium levels should be monitored as a guide to dosage. Using aseptic technique, all or part of the contents of one or more vials may be added to other intravenous fluids to provide any desired number of millimoles (mM) of phosphorus.
In patients on total parenteral nutrition, approximately 12 to 15 mM of phosphorus (equivalent to 372 to 465 mg elemental phosphorus) per liter bottle of TPN solution containing 250 g dextrose is usually adequate to maintain normal serum phosphorus, though larger amounts may be required in hypermetabolic states. The amount of sodium and phosphorus which accompanies the addition of sodium phosphate also should be kept in mind, and if necessary, serum sodium levels should be monitored.
The suggested dose of phosphorus for infants receiving TPN is 1.5 to 2 mM P/kg/day.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
HOW SUPPLIED
Sodium Phosphates Injection, USP, 3 mM P/mL is supplied as per below.
| Unit of Sale | Concentration | Unit of Use |
|---|---|---|
| NDC 0409-7391-72
Tray containing 25 vials | 45 mM (3 mM P/mL) containing 60 mEq Na+ (4.0 mEq/mL) | NDC 0409-7391-82 15 mL Single-dose Plastic Vial |
Each container is partially filled to provide air space needed for complete vacuum withdrawal of the contents into the I.V. container.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]

Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1044-3.0
01/2018
PRINCIPAL DISPLAY PANEL - 15 mL Vial Label
PRINCIPAL DISPLAY PANEL - 15 mL Vial Tray
INGREDIENTS AND APPEARANCE
| SODIUM PHOSPHATES
sodium phosphate, monobasic, monohydrate and sodium phosphate, dibasic, anhydrous injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 093132819 | ANALYSIS(0409-7391) , LABEL(0409-7391) , MANUFACTURE(0409-7391) , PACK(0409-7391) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 827731089 | ANALYSIS(0409-7391) | |