Search by Drug Name or NDC
NDC 00641-6018-01 Dopram 20 mg/mL Details
Dopram 20 mg/mL
Dopram is a INTRAVENOUS INJECTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Hikma Pharmaceuticals USA Inc.. The primary component is DOXAPRAM HYDROCHLORIDE.
Product Information
| NDC | 00641-6018 |
|---|---|
| Product ID | 0641-6018_3860168c-32e9-4151-b08e-25d4bbac75b6 |
| Associated GPIs | 61300020102005 |
| GCN Sequence Number | 003533 |
| GCN Sequence Number Description | doxapram HCl VIAL 20 MG/ML INTRAVEN |
| HIC3 | H2A |
| HIC3 Description | CENTRAL NERVOUS SYSTEM STIMULANTS |
| GCN | 12520 |
| HICL Sequence Number | 001543 |
| HICL Sequence Number Description | DOXAPRAM HCL |
| Brand/Generic | Brand |
| Proprietary Name | Dopram |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Doxapram hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 20 |
| Active Ingredient Units | mg/mL |
| Substance Name | DOXAPRAM HYDROCHLORIDE |
| Labeler Name | Hikma Pharmaceuticals USA Inc. |
| Pharmaceutical Class | Increased Medullary Respiratory Drive [PE], Respiratory Stimulant [EPC] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA014879 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 00641-6018-01 (00641601801)
| NDC Package Code | 0641-6018-01 |
|---|---|
| Billing NDC | 00641601801 |
| Package | 20 mL in 1 VIAL (0641-6018-01) |
| Marketing Start Date | 1965-06-23 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b8eb330a-a75b-46cb-b694-5be9327ed2eb Details
DESCRIPTION
DOPRAM Injection (doxapram hydrochloride injection, USP) is a clear, colorless, sterile, non-pyrogenic, aqueous solution with pH 3.5 to 5, for intravenous administration.
Each 1 mL contains:
Doxapram Hydrochloride, USP ................................................................. 20 mg
Benzyl Alcohol, NF (as preservative) ......................................................... 0.9%
Water for Injection, USP ...........…................................................................. q.s.
Doxapram Injection is a respiratory stimulant.
Doxapram hydrochloride is a white to off-white, crystalline powder, sparingly soluble in water, alcohol and chloroform. Chemically, doxapram hydrochloride is 1-ethyl-4-[2-(4-morpholinyl)ethyl]-3,3-diphenyl-2-pyrrolidinone monohydrochloride, monohydrate.
The chemical structure is:
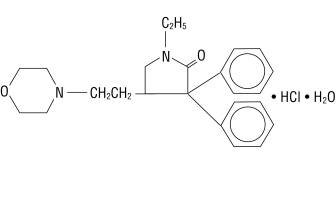
C24H31ClN2O2 • H2O M.W. 432.98
CLINICAL PHARMACOLOGY
Pharmacodynamics
Doxapram hydrochloride produces respiratory stimulation mediated through the peripheral carotid chemoreceptors. As the dosage level is increased, the central respiratory centers in the medulla are stimulated with progressive stimulation of other parts of the brain and spinal cord.
The onset of respiratory stimulation following the recommended single intravenous injection of doxapram hydrochloride usually occurs in 20 to 40 seconds with peak effect at 1 to 2 minutes. The duration of effect may vary from 5 to 12 minutes.
The respiratory stimulant action is manifested by an increase in tidal volume associated with a slight increase in respiratory rate.
A pressor response may result following doxapram administration. Provided there is no impairment of cardiac function, the pressor effect is more marked in hypovolemic than in normovolemic states. The pressor response is due to the improved cardiac output rather than peripheral vasoconstriction. Following doxapram administration, an increased release of catecholamines has been noted.
Although opiate-induced respiratory depression is antagonized by doxapram, the analgesic effect is not affected.
INDICATIONS AND USAGE
Postanesthesia
- When the possibility of airway obstruction and/or hypoxia have been eliminated, doxapram may be used to stimulate respiration in patients with drug-induced postanesthesia respiratory depression or apnea other than that due to muscle relaxant drugs.
- To pharmacologically stimulate deep breathing in the postoperative patient. (A quantitative method of assessing oxygenation, such as pulse oximetry, is recommended.)
Drug-Induced Central Nervous System Depression
Exercising care to prevent vomiting and aspiration, doxapram may be used to stimulate respiration, hasten arousal, and to encourage the return of laryngopharyngeal reflexes in patients with mild to moderate respiratory and CNS depression due to drug overdosage.
Chronic Pulmonary Disease Associated with Acute Hypercapnia
Doxapram is indicated as a temporary measure in hospitalized patients with acute respiratory insufficiency superimposed on chronic obstructive pulmonary disease. Its use should be for a short period of time (see DOSAGE AND ADMINISTRATION) as an aid in the prevention of elevation of arterial CO2 tension during the administration of oxygen.
It should not be used in conjunction with mechanical ventilation.
CONTRAINDICATIONS
Doxapram is contraindicated in patients with known hypersensitivity to the drug or any of the injection components.
Doxapram should not be used in patients with epilepsy or other convulsive disorders.
Doxapram is contraindicated in patients with proven or suspected pulmonary embolism.
Doxapram is contraindicated in patients with mechanical disorders of ventilation such as mechanical obstruction, muscle paresis (including neuromuscular blockade), flail chest, pneumothorax, acute bronchial asthma, pulmonary fibrosis, or other conditions resulting in restriction of the chest wall, muscles of respiration, or alveolar expansion.
Doxapram is contraindicated in patients with evidence of head injury, cerebral vascular accident, or cerebral edema, and in those with significant cardiovascular impairment, uncompensated heart failure, severe coronary artery disease, or severe hypertension, including that associated with hyperthyroidism or pheochromocytoma. (See WARNINGS.)
WARNINGS
Doxapram should not be used in conjunction with mechanical ventilation.
Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The amount of benzyl alcohol from medications is usually considered negligible compared to that received in flush solutions containing benzyl alcohol. Administration of high dosages of medications containing this preservative must take into account the total amount of benzyl alcohol administered. The amount of benzyl alcohol at which toxicity may occur is not known. If the patient requires more than the recommended dosages or other medications containing this preservative, the practitioner must consider the daily metabolic load of benzyl alcohol from these combined sources (see PRECAUTIONS, Pediatric Use).
In Postanesthetic Use
- Doxapram is neither an antagonist to muscle relaxant drugs nor a specific narcotic antagonist. More specific tests (eg, peripheral nerve stimulation, airway pressures, head lift, pulse oximetry, and end-tidal carbon dioxide) to assess adequacy of ventilation are recommended before administering doxapram.
- Doxapram should be administered with great care and only under careful supervision to patients with hypermetabolic states such as hyperthyroidism or pheochromocytoma.
- Since narcosis may recur after stimulation with doxapram, care should be taken to maintain close observation until the patient has been fully alert for ½ to 1 hour.
- In patients who have received general anesthesia utilizing a volatile agent known to sensitize the myocardium to catecholamines, administration of doxapram should be delayed until the volatile agent has been excreted in order to lessen the potential for arrhythmias, including ventricular tachycardia and ventricular fibrillation (see PRECAUTIONS, Drug Interactions).
In Drug-Induced CNS and Respiratory Depression
Doxapram alone may not stimulate adequate spontaneous breathing or provide sufficient arousal in patients who are severely depressed either due to respiratory failure or to CNS depressant drugs, but may be used as an adjunct to established supportive measures and resuscitative techniques.
PRECAUTIONS
General
- An adequate airway is essential and airway protection should be considered since doxapram may stimulate vomiting.
- Recommended dosages of doxapram should be employed and maximum total dosages should not be exceeded. In order to avoid side effects, it is advisable to use the minimum effective dosage.
- Monitoring of the blood pressure, pulse rate, and deep tendon reflexes is recommended to prevent overdosage.
- Vascular extravasation or use of a single injection site over an extended period should be avoided since either may lead to thrombophlebitis or local skin irritation.
- Rapid infusion may result in hemolysis.
- Lowered pCO2 induced by hyperventilation produces cerebral vasoconstriction and slowing of the cerebral circulation. This should be taken into consideration on an individual basis. In certain patients a pressor effect of doxapram on the pulmonary circulation may result in a fall of the arterial pO2 probably due to a worsening of ventilation perfusion-matching in the lungs despite an overall improvement in alveolar ventilation and a fall in pCO2. Patients should be carefully supervised taking into account available blood gas measurements.
- There is a risk that doxapram will produce adverse effects (including seizures) due to general central nervous system stimulation. Muscle involvement may range from fasciculation to spasticity. Anticonvulsants such as intravenous short-acting barbiturates, along with oxygen and resuscitative equipment should be readily available to manage overdosage manifested by excessive central nervous system stimulation. Slow administration of the drug and careful observation of the patient during administration and for some time subsequently are advisable. These precautions are to assure that the protective reflexes have been restored and to prevent possible post-hyperventilation or hypoventilation.
- Doxapram should be administered cautiously to patients receiving sympathomimetic or monoamine oxidase inhibiting drugs, since an additive pressor effect may occur.
- Blood pressure increases are generally modest but significant increases have been noted in some patients. Because of this, doxapram is not recommended for use in patients with severe hypertension (see CONTRAINDICATIONS).
- Cardiovascular effects may include various dysrhythmias. Patients receiving doxapram should be monitored for disturbance of their cardiac rhythm.
- If sudden hypotension or dyspnea develops, doxapram should be stopped.
- Doxapram should be administered with caution to patients with significantly impaired hepatic or renal function as a reduction in the rate of metabolism or excretion of metabolites may alter the response.
In Postanesthetic Use
- The same consideration to pre-existing disease states should be exercised as in non-anesthetized individuals. See CONTRAINDICATIONS and WARNINGS covering use in hypertension, asthma, disturbances of respiratory mechanics including airway obstruction, CNS disorders including increased cerebrospinal fluid pressure, convulsive disorders, acute agitation, and profound metabolic disorders.
- See PRECAUTIONS, Drug Interactions.
In Chronic Obstructive Pulmonary Disease
- Arrhythmias seen in some patients in acute respiratory failure secondary to chronic obstructive pulmonary disease are probably the result of hypoxia. Doxapram should be used with caution in these patients.
- Arterial blood gases should be drawn prior to the initiation of doxapram infusion and oxygen administration, then at least every ½ hour during the infusion period to prevent development of CO2 retention and acidosis in patients with chronic obstructive pulmonary disease with acute hypercapnia. Doxapram administration does not diminish the need for careful monitoring of the patient or the need for supplemental oxygen in patients with acute respiratory failure. Doxapram should be stopped if the arterial blood gases deteriorate, and mechanical ventilation should be initiated.
Drug Interactions
Administration of doxapram to patients who are receiving sympathomimetic or monoamine oxidase inhibiting drugs may result in an additive pressor effect (see PRECAUTIONS, General).
In patients who have received neuromuscular blocking agents, doxapram may temporarily mask the residual effects of these drugs.
In patients who have received general anesthesia utilizing a volatile agent known to sensitize the myocardium to catecholamines, administration of doxapram should be delayed until the volatile agent has been excreted in order to lessen the potential for arrhythmias, including ventricular tachycardia and ventricular fibrillation (see WARNINGS).
There may be an interaction between doxapram and aminophylline and between doxapram and theophylline manifested by increased skeletal muscle activity, agitation, and hyperactivity.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenic or mutagenic studies have been performed using doxapram. Doxapram did not adversely affect the breeding performance of rats.
Pregnancy
PREGNANCY CATEGORY B
Reproduction studies have been performed in rats at doses up to 1.6 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to doxapram. There are, however, no adequate and well-controlled studies in pregnant women. Because the animals in the reproduction studies were dosed by the IM and oral routes and animal reproduction studies, in general, are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when doxapram hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 years have not been established. This product contains benzyl alcohol as a preservative. Benzyl alcohol, a component of this product, has been associated with serious adverse events and death, particularly in pediatric patients. The “gasping syndrome”, (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates and low-birth-weight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Although normal therapeutic doses of this product deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the “gasping syndrome”, the minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low-birth-weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
Premature neonates given doxapram have developed hypertension, irritability, jitteriness, hyperglycemia, glucosuria, abdominal distension, increased gastric residuals, vomiting, bloody stools, necrotizing enterocolitis, erratic limb movements, excessive crying, disturbed sleep, premature eruption of teeth, and QT prolongation that has resulted in heart block. In premature neonates with risk factors such as a previous seizure, perinatal asphyxia, or intracerebral hemorrhage, seizures have occurred. In many instances, doxapram was administered following administration of xanthine derivatives such as caffeine, aminophylline or theophylline.
ADVERSE REACTIONS
Adverse reactions reported coincident with the administration of DOPRAM (doxapram hydrochloride, USP) include:
1. Central and Autonomic Nervous Systems
Pyrexia, flushing, sweating; pruritus and paresthesia, such as a feeling of warmth, burning, or hot sensation, especially in the area of genitalia and perineum; apprehension, disorientation, pupillary dilatation, hallucinations, headache, dizziness, hyperactivity, involuntary movements, muscle spasticity, muscle fasciculations, increased deep tendon reflexes, clonus, bilateral Babinski, and convulsions.
2. Respiratory
Dyspnea, cough, hyperventilation, tachypnea, laryngospasm, bronchospasm, hiccough, and rebound hypoventilation.
3. Cardiovascular
Phlebitis, variations in heart rate, lowered T-waves, arrhythmias (including ventricular tachycardia and ventricular fibrillation), chest pain, tightness in chest. A mild to moderate increase in blood pressure is commonly noted and may be of concern in patients with severe cardiovascular diseases.
5. Genitourinary
Stimulation of urinary bladder with spontaneous voiding; urinary retention. Elevation of BUN and albuminuria.
6. Hemic and Lymphatic
Hemolysis with rapid infusion. A decrease in hemoglobin, hematocrit, or red blood cell count has been observed in postoperative patients. In the presence of pre-existing leukopenia, a further decrease in WBC has been observed following anesthesia and treatment with doxapram hydrochloride.
OVERDOSAGE
Signs and Symptoms
Symptoms of overdosage are extensions of the pharmacologic effects of the drug. Excessive pressor effect, such as hypertension, tachycardia, skeletal muscle hyperactivity, and enhanced deep tendon reflexes may be early signs of overdosage. Therefore, the blood pressure, pulse rate, and deep tendon reflexes should be evaluated periodically and the dosage or infusion rate adjusted accordingly.
Other effects may include agitation, confusion, sweating, cough, and dyspnea.
Convulsive seizures are unlikely at recommended dosages. In unanesthetized animals, the convulsant dose is 70 times greater than the respiratory stimulant dose. Intravenous LD50 values in the mouse and rat were approximately 75 mg/kg and in the cat and dog were 40 to 80 mg/kg.
Except for management of chronic obstructive pulmonary disease associated with acute hypercapnia, the maximum recommended dosage is 3 GRAMS/24 HOURS. (See DOSAGE AND ADMINISTRATION.)
Management
There is no specific antidote for doxapram. Management should be symptomatic. Anticonvulsants, along with oxygen and resuscitative equipment should be readily available to manage overdosage manifested by excessive central nervous system stimulation. Slow administration of the drug and careful observation of the patient during administration and for some time subsequently are advisable. These precautions are to assure that the protective reflexes have been restored and to prevent possible post-hyperventilation or hypoventilation.
There is no evidence that doxapram is dialyzable; further, the half-life of doxapram makes it unlikely that dialysis would be appropriate in managing overdose with this drug.
DOSAGE AND ADMINISTRATION
NOTE: CONTAINS BENZYL ALCOHOL (see PRECAUTIONS)
In Postanesthetic Use
|
|||
| I.V. Administration | Recommended Dosage mg/kg | Maximum dose per single injection mg/kg | Maximum total dose* mg/kg |
| Single Injection | 0.5-1 | 1.5 | 1.5 |
| Repeat Injections (5 min. intervals) | 0.5-1 | 1.5 | 2 |
| Infusion | 0.5-1 | – | 4 |
BY I.V. INJECTION
(See Table I. Dosage for postanesthetic use—I.V.)
The recommended dose for I.V. administration is 0.5 – 1 mg/kg for a single injection and at 5-minute intervals. Careful observation of the patient during administration and for some time subsequently are advisable. The maximum total dosage by I.V. injection is 2 mg/kg.
BY INFUSION
The solution is prepared by adding 250 mg of doxapram (12.5 mL) to 250 mL of dextrose 5% or 10% in water or normal saline solution. The infusion is initiated at a rate of approximately 5 mg/minute until a satisfactory respiratory response is observed, and maintained at a rate of 1 to 3 mg/minute. The rate of infusion should be adjusted to sustain the desired level of respiratory stimulation with a minimum of side effects. The maximum total dosage by infusion is 4 mg/kg, or approximately 300 mg for the average adult.
In the Management of Drug-Induced CNS Depression
(See Table II. Dosage for drug-induced CNS depression.)
|
||
| METHOD ONE Priming dose single/repeat I.V. Injection | METHOD TWO Rate of Intermittent I.V. Infusion |
|
| Level of Depression | mg/kg | mg/kg/hr |
| Mild* | 1 | 1-2 |
| Moderate† | 2 | 2-3 |
METHOD ONE
Using Single and/or Repeat Single I.V. Injections
- Give priming dose of 2 mg/kg body weight and repeat in 5 minutes. The priming dose for moderate depression is 2 mg/kg and the priming dose for mild depression is 1 mg/kg.
- Repeat same dose q 1 to 2h until patient wakens. Watch for relapse into unconsciousness or development of respiratory depression, since DOPRAM does not affect the metabolism of CNS-depressant drugs.
- If relapse occurs, resume injections q 1 to 2h until arousal is sustained, or total maximum daily dose (3 grams) is given. After maximum dose has been given (3 grams), allow patient to sleep until 24 hours have elapsed from first injection of DOPRAM, using assisted or automatic respiration if necessary.
- Repeat procedure the following day until patient breathes spontaneously and sustains desired level of consciousness, or until maximum dosage (3 grams) is given.
- Repetitive doses should be administered only to patients who have shown response to the initial dose.
- Failure to respond appropriately indicates the need for neurologic evaluation for a possible central nervous system source of sustained coma.
METHOD TWO
By Intermittent I.V. Infusion
- Give priming dose as in Method One.
- If patient wakens, watch for relapse; if no response, continue general supportive treatment for 1 to 2 hours and repeat priming dose of DOPRAM. If some respiratory stimulation occurs, prepare I.V. infusion by adding 250 mg of DOPRAM (12.5 mL) to 250 mL of saline or dextrose solution. Deliver at rate of 1 to 3 mg/min (60 to 180 mL/hr) according to size of patient and depth of coma. Discontinue DOPRAM if patient begins to waken or at end of 2 hours.
- Continue supportive treatment for ½ to 2 hours and repeat Step b.
- Do not exceed 3 grams/day.
Chronic Obstructive Pulmonary Disease Associated with Acute Hypercapnia
- One vial of doxapram (400 mg) should be mixed with 180 mL of dextrose 5% or 10% or normal saline solution (concentration of 2 mg/mL). The infusion should be started at 1 to 2 mg/minute (½ to 1 mL/minute); if indicated, increase to a maximum of 3 mg/minute. Arterial blood gases should be determined prior to the onset of doxapram’s administration and at least every half hour during the two hours of infusion to insure against the insidious development of CO2-RETENTION AND ACIDOSIS. Alteration of oxygen concentration or flow rate may necessitate adjustment in the rate of doxapram infusion.
- Predictable blood gas patterns are more readily established with a continuous infusion of doxapram. If the blood gases show evidence of deterioration, the infusion of doxapram should be discontinued.
- ADDITIONAL INFUSIONS BEYOND THE SINGLE MAXIMUM TWO HOUR ADMINISTRATION PERIOD ARE NOT RECOMMENDED.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Diluent Compatibility
Doxapram hydrochloride is compatible with 5% and 10% dextrose in water or normal saline.
Incompatibility
ADMIXTURE OF DOXAPRAM WITH ALKALINE SOLUTIONS SUCH AS 2.5% THIOPENTAL SODIUM, SODIUM BICARBONATE, FUROSEMIDE, OR AMINOPHYLLINE WILL RESULT IN PRECIPITATION OR GAS FORMATION.
Doxapram is also not compatible with ascorbic acid, cefoperazone sodium, cefotaxime sodium, cefotetan sodium, cefuroxime sodium, folic acid, dexamethasone disodium phosphate, diazepam, hydrocortisone sodium phosphate, methylprednisolone sodium, or hydrocortisone sodium succinate.
Admixture of doxapram and ticarcillin disodium results in an 18% loss of doxapram in 3 hours. When doxapram is mixed with minocycline hydrochloride, there is a loss of 8% of doxapram in 3 hours and a 13% loss of doxapram in 6 hours.
HOW SUPPLIED
DOPRAM Injection (doxapram hydrochloride injection, USP) is available in cartons of one 20 mL multiple dose vial containing 20 mg of doxapram hydrochloride per mL with benzyl alcohol 0.9% as the preservative (NDC 0641-6018-01).
Store at Controlled Room Temperature, Between 20˚C to 25˚C (68˚F to 77˚F). See USP.
SPL UNCLASSIFIED SECTION
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
Manufactured by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
Revised June 2020
462-175-07
PACKAGE LABELING - PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| DOPRAM
doxapram hydrochloride injection |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Hikma Pharmaceuticals USA Inc. (946499746) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hikma Pharmaceuticals USA Inc. | 946499746 | ANALYSIS(0641-6018) , LABEL(0641-6018) , MANUFACTURE(0641-6018) | |