Search by Drug Name or NDC
NDC 41250-0111-35 Meijer Enema 7; 19 g/118mL; g/118mL Details
Meijer Enema 7; 19 g/118mL; g/118mL
Meijer Enema is a RECTAL ENEMA in the HUMAN OTC DRUG category. It is labeled and distributed by Meijer Distribution Inc. The primary component is SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM; SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM.
Product Information
| NDC | 41250-0111 |
|---|---|
| Product ID | 41250-111_b11cce22-3ca0-4d5d-8844-7fd1b42c1ce6 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Meijer Enema |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Saline Laxative |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | ENEMA |
| Route | RECTAL |
| Active Ingredient Strength | 7; 19 |
| Active Ingredient Units | g/118mL; g/118mL |
| Substance Name | SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM; SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM |
| Labeler Name | Meijer Distribution Inc |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part334 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
NDC 41250-0111-35 (41250011135)
| NDC Package Code | 41250-111-35 |
|---|---|
| Billing NDC | 41250011135 |
| Package | 1 BOTTLE in 1 CARTON (41250-111-35) / 133 mL in 1 BOTTLE |
| Marketing Start Date | 2013-05-10 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 2c82d239-261e-4e3d-8a90-caecb527bed3 Details
SPL UNCLASSIFIED SECTION
Use
Warnings
For rectal use only.
Ask a doctor before use if you
- have already used a laxative for more than 3 days
- have kidney disease, have heart problems, or are dehydrated
- are 55 years of age or older
- are on a sodium-restricted diet
- have abdominal pain, nausea, or vomiting
- have a sudden change in bowel habits lasting more than 2 weeks
Ask a doctor or pharmacist before use if you are taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
When using this product
- do not use more than directed. Serious side effects may occur from excess dosage
- do not use for more than 3 days, without asking a doctor
Directions (or as directed by a doctor)
Single daily dosage (per 24 hours)
Do not use if taking another sodium phosphate product.
Do not use more unless directed by a doctor. See Warnings.
| Adults and children 12 years of age and older | 1 bottle once daily |
| Children 2 to under 12 years of age | Use Pediatric Enema |
| Children under 2 years of age | DO NOT USE |
Other information
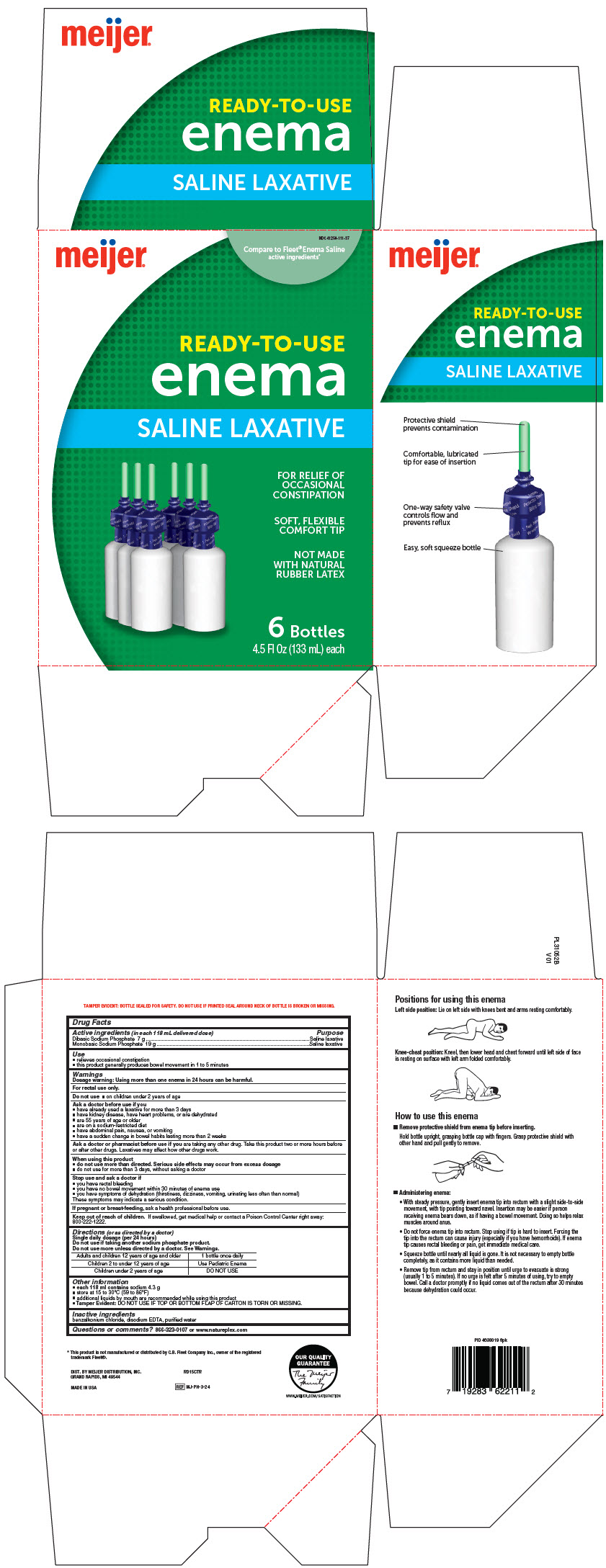
PRINCIPAL DISPLAY PANEL - 133 mL Bottle Carton
INGREDIENTS AND APPEARANCE
| MEIJER ENEMA
saline laxative enema |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Meijer Distribution Inc (006959555) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Natureplex LLC | 062808196 | MANUFACTURE(41250-111) | |
Revised: 1/2020
Document Id: b11cce22-3ca0-4d5d-8844-7fd1b42c1ce6
Set id: 2c82d239-261e-4e3d-8a90-caecb527bed3
Version: 5
Effective Time: 20200131