Search by Drug Name or NDC
NDC 43598-0975-58 DEXMEDETOMIDINE HYDROCHLORIDE 4 ug/mL Details
DEXMEDETOMIDINE HYDROCHLORIDE 4 ug/mL
DEXMEDETOMIDINE HYDROCHLORIDE is a INTRAVENOUS INJECTION, SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Dr.Reddy's Laboratories Inc. The primary component is DEXMEDETOMIDINE HYDROCHLORIDE.
Product Information
| NDC | 43598-0975 |
|---|---|
| Product ID | 43598-975_079da288-33ea-0240-9d07-bc04c118919b |
| Associated GPIs | 60206030202040 |
| GCN Sequence Number | 070877 |
| GCN Sequence Number Description | dexmedetomidine in 0.9 % NaCl INFUS. BTL 400MCG/100 INTRAVEN |
| HIC3 | H2E |
| HIC3 Description | SEDATIVE-HYPNOTICS,NON-BARBITURATE |
| GCN | 34539 |
| HICL Sequence Number | 040230 |
| HICL Sequence Number Description | DEXMEDETOMIDINE HCL IN 0.9 % SODIUM CHLORIDE |
| Brand/Generic | Generic |
| Proprietary Name | DEXMEDETOMIDINE HYDROCHLORIDE |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | DEXMEDETOMIDINE HYDROCHLORIDE |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, SOLUTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 4 |
| Active Ingredient Units | ug/mL |
| Substance Name | DEXMEDETOMIDINE HYDROCHLORIDE |
| Labeler Name | Dr.Reddy's Laboratories Inc |
| Pharmaceutical Class | Adrenergic alpha2-Agonists [MoA], Central alpha-2 Adrenergic Agonist [EPC], General Anesthesia [PE] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA209307 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

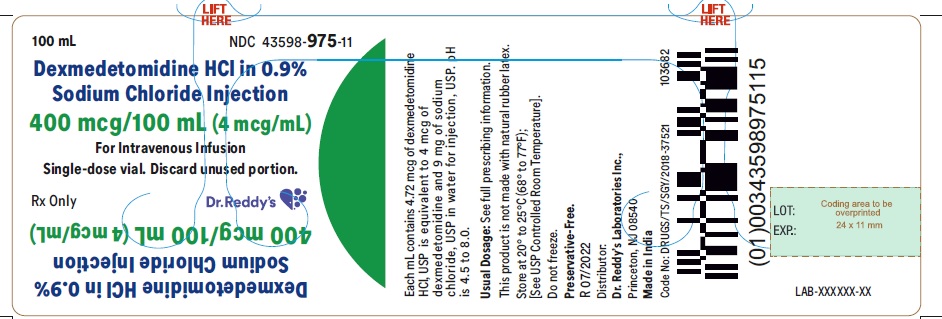


NDC 43598-0975-58 (43598097558)
| NDC Package Code | 43598-975-58 |
|---|---|
| Billing NDC | 43598097558 |
| Package | 10 VIAL in 1 CARTON (43598-975-58) / 100 mL in 1 VIAL (43598-975-11) |
| Marketing Start Date | 2020-08-17 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL d174c19d-56f1-4ef4-087c-23c26db8e40b Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
DEXMEDETOMIDINE HYDROCHLORIDE in 0.9% SODIUM CHLORIDE injection, for intravenous use.
Initial U.S. Approval: 1999
INDICATIONS AND USAGE
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is a relatively selective alpha2-adrenergic agonist indicated for:
- Sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting. Administer dexmedetomidine hydrochloride in 0.9% sodium chloride injection by continuous infusion not to exceed 24 hours. (1.1).
- Sedation of non-intubated patients prior to and/or during surgical and other procedures. (1.2)
DOSAGE AND ADMINISTRATION
- Individualize and titrate dexmedetomidine hydrochloride in 0.9% sodium chloride injection dosing to desired clinical effect. (2.1)
- Administer dexmedetomidine hydrochloride in 0.9% sodium chloride injection using a controlled infusion device. (2.1)
- The 200 mcg/50mL and 400 mcg/100 mL single-dose Vials do not require further dilution prior to administration. (2.4)
For Adult Intensive Care Unit Sedation: Generally initiate at one mcg/kg over 10 minutes, followed by a maintenance infusion of 0.2 to 0.7 mcg/kg/hour. (2.2)
For Adult Procedural Sedation: Generally initiate at one mcg/kg over 10 minutes, followed by a maintenance infusion initiated at 0.6 mcg/kg/hour and titrated to achieve desired clinical effect with doses ranging from 0.2 to 1 mcg/kg/hour. (2.2)
Alternative Doses: Recommended for patients over 65 years of age and awake fiberoptic intubation patients. (2.2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Monitoring: Continuously monitor patients while receiving dexmedetomidine hydrochloride in 0.9% sodium chloride injection (5.1)
- Bradycardia and Sinus Arrest: Have occurred in young healthy volunteers with high vagal tone or with different routes of administration, e.g., rapid intravenous or bolus administration. (5.2) Hypotension and Bradycardia: May necessitate medical intervention. May be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension, and in the elderly. Use with caution in patients with advanced heart block or severe ventricular dysfunction. (5.2)
- Co-administration with Other Vasodilators or Negative Chronotropic Agents: Use with caution due to additive pharmacodynamic effects. (5.2)
- Transient Hypertension: Observed primarily during the loading dose. Consider reduction in loading infusion rate. (5.3)
- Arousability: Patients can become aroused/alert with stimulation; this alone should not be considered as lack of efficacy. (5.4)
- Tolerance and Tachyphylaxis: Prolonged exposure to dexmedetomidine beyond 24 hours may be associated with tolerance and tachyphylaxis and a dose-related increase in adverse events. (5.6)
ADVERSE REACTIONS
- The most common adverse reactions (incidence >2%) are hypotension, bradycardia, and dry mouth. (6.1)
- Adverse reactions associated with infusions >24 hours in duration include ARDS, respiratory failure, and agitation.(6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Dr. REDDY’S LABORATORIES Inc., at 1-888-375-3784 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
Anesthetics, Sedatives, Hypnotics, and Opioids: Enhancement of pharmacodynamic effects. Reduction in dosage of dexmedetomidine hydrochloride in 0.9% sodium chloride injection or the concomitant medication may be required. (7.1)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Nursing Mothers: Caution should be exercised when administered to a nursing woman. (8.3)
- Geriatric Patients: Dose reduction should be considered. (2.2, 2.3, 5.2, 8.5)
- Hepatic Impairment: Dose reduction should be considered. (2.2, 2.3, 5.7, 8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Intensive Care Unit Sedation
1.2 Procedural Sedation
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Guidelines
2.2 Dosage Information
2.3 Dosage Adjustment
2.4 Preparation of Solution
2.5 Administration with Other Fluids
2.6 Compatibility with Natural Rubber
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Drug Administration
5.2 Hypotension, Bradycardia, and Sinus Arrest
5.3 Transient Hypertension
5.4 Arousability
5.5 Withdrawal
5.6 Tolerance and Tachyphylaxis
5.7 Hepatic Impairment
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Anesthetics, Sedatives, Hypnotics, Opioids
7.2 Neuromuscular Blockers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
13.2 Animal Pharmacology and/or Toxicology
14 CLINICAL STUDIES
14.1 Intensive Care Unit Sedation
14.2 Procedural Sedation
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
1.1 Intensive Care Unit Sedation
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is indicated for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting. Dexmedetomidine hydrochloride in 0.9% sodium chloride injection should be administered by continuous infusion not to exceed 24 hours.
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection has been continuously infused in mechanically ventilated patients prior to extubation, during extubation, and post-extubation. It is not necessary to discontinue dexmedetomidine hydrochloride in 0.9% sodium chloride injection prior to extubation.
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Guidelines
- Dexmedetomidine hydrochloride in 0.9% sodium chloride injection dosing should be individualized and titrated to desired clinical response.
- Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is not indicated for infusions lasting longer than 24 hours.
- Dexmedetomidine hydrochloride in 0.9% sodium chloride injection should be administered using a controlled infusion device.
2.2 Dosage Information
Table 1: Dosage Information
| INDICATION | DOSAGE AND ADMINISTRATION | |
| Initiation of Intensive Care Unit Sedation | For adult patients: a loading infusion of one mcg/kg over 10 minutes. For adult patients being converted from alternate sedative therapy: a loading dose may not be required. For patients over 65 years of age: a dose reduction should be considered [see Use in Specific Populations (8.5)]. For adult patients with impaired hepatic function: a dose reduction should be considered [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. |
|
| Maintenance of Intensive Care Unit Sedation | For adult patients: a maintenance infusion of 0.2 to 0.7 mcg/kg/hour. The rate of the maintenance infusion should be adjusted to achieve the desired level of sedation. For patients over 65 years of age: a dose reduction should be considered [see Use in Specific Populations (8.5)]. For adult patients with impaired hepatic function: a dose reduction should be considered [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. |
|
| Initiation of Procedural Sedation | For adult patients: a loading infusion of one mcg/kg over 10 minutes. For less invasive procedures such as ophthalmic surgery, a loading infusion of 0.5 mcg/kg given over 10 minutes may be suitable. For awake fiberoptic intubation in adult patients: a loading infusion of one mcg/kg over 10 minutes. For patients over 65 years of age: a loading infusion of 0.5 mcg/kg over 10 minutes [see Use in Specific Populations (8.5)]. For adult patients with impaired hepatic function: a dose reduction should be considered [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. |
|
| Maintenance of Procedural Sedation | For adult patients: the maintenance infusion is generally initiated at 0.6 mcg/kg/hour and titrated to achieve desired clinical effect with doses ranging from 0.2 to 1 mcg/kg/hour. The rate of the maintenance infusion should be adjusted to achieve the targeted level of sedation. For awake fiberoptic intubation in adult patients: a maintenance infusion of 0.7 mcg/kg/hour is recommended until the endotracheal tube is secured. For patients over 65 years of age: a dose reduction should be considered [see Use in Specific Populations (8.5)]. For adult patients with impaired hepatic function: a dose reduction should be considered [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. |
2.3 Dosage Adjustment
Due to possible pharmacodynamic interactions, a reduction in dosage of dexmedetomidine hydrochloride in 0.9% sodium chloride injection or other concomitant anesthetics, sedatives, hypnotics or opioids may be required when co-administered [see Drug Interactions (7.1)].
Dosage reductions may need to be considered for adult patients with hepatic impairment and geriatric patients [see Warnings and Precautions (5.7), Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
2.4 Preparation of Solution
Strict aseptic technique must always be maintained during handling of dexmedetomidine hydrochloride in 0.9% sodium chloride injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection, 200 mcg/50 mL (4 mcg/mL) and 400 mcg/100 mL (4 mcg/mL)
Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection is supplied in glass containers containing a premixed, ready to use dexmedetomidine hydrochloride solution in 0.9% sodium chloride in water. No further dilution of these preparations are necessary.
2.5 Administration with Other Fluids
Dexmedetomidine hydrochloride in 0.9% sodium chloride infusion should not be co-administered through the same intravenous catheter with blood or plasma because physical compatibility has not been established.
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection has been shown to be incompatible when administered with the following drugs: amphotericin B, diazepam.
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection has been shown to be compatible when administered with the following intravenous fluids:
- 0.9% sodium chloride in water
- 5% dextrose in water
- 20% mannitol
- Lactated Ringer’s solution
- 100 mg/mL magnesium sulfate solution
- 0.3% potassium chloride solution
2.6 Compatibility with Natural Rubber
Compatibility studies have demonstrated the potential for absorption of dexmedetomidine hydrochloride in 0.9% sodium chloride injection to some types of natural rubber. Although dexmedetomidine hydrochloride in 0.9% sodium chloride injection is dosed to effect, it is advisable to use administration components made with synthetic or coated natural rubber gaskets.
3 DOSAGE FORMS AND STRENGTHS
Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection is clear and colorless and is available as follows.
Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection, 200 mcg dexmedetomidine/50 mL (4 mcg/ mL) in a glass vial. Ready to use.
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection, 400 mcg dexmedetomidine/100 mL (4 mcg/ mL) in a glass vial. Ready to use.
5 WARNINGS AND PRECAUTIONS
5.1 Drug Administration
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection should be administered only by persons skilled in the management of patients in the intensive care or operating room setting. Due to the known pharmacological effects of dexmedetomidine, patients should be continuously monitored while receiving dexmedetomidine hydrochloride in 0.9% sodium chloride injection.
5.2 Hypotension, Bradycardia, and Sinus Arrest
Clinically significant episodes of bradycardia and sinus arrest have been reported with dexmedetomidine administration in young, healthy adult volunteers with high vagal tone or with different routes of administration including rapid intravenous or bolus administration.
Reports of hypotension and bradycardia have been associated with dexmedetomidine infusion. Some of these cases have resulted in fatalities. If medical intervention is required, treatment may include decreasing or stopping the infusion of dexmedetomidine hydrochloride in 0.9% sodium chloride injection, increasing the rate of intravenous fluid administration, elevation of the lower extremities, and use of pressor agents. Because dexmedetomidine has the potential to augment bradycardia induced by vagal stimuli, clinicians should be prepared to intervene. The intravenous administration of anticholinergic agents (e.g., glycopyrrolate, atropine) should be considered to modify vagal tone. In clinical trials, glycopyrrolate or atropine were effective in the treatment of most episodes of dexmedetomidine-induced bradycardia. However, in some patients with significant cardiovascular dysfunction, more advanced resuscitative measures were required.
Caution should be exercised when administering dexmedetomidine hydrochloride in 0.9% sodium chloride injection to patients with advanced heart block and/or severe ventricular dysfunction. Because dexmedetomidine decreases sympathetic nervous system activity, hypotension and/or bradycardia may be expected to be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension and in elderly patients.
In clinical trials where other vasodilators or negative chronotropic agents were co-administered with dexmedetomidine hydrochloride in 0.9% sodium chloride injection an additive pharmacodynamic effect was not observed. Nonetheless, caution should be used when such agents are administered concomitantly with dexmedetomidine hydrochloride in 0.9% sodium chloride injection.
5.3 Transient Hypertension
Transient hypertension has been observed primarily during the loading dose in association with the initial peripheral vasoconstrictive effects of dexmedetomidine hydrochloride in 0.9% sodium chloride injection. Treatment of the transient hypertension has generally not been necessary, although reduction of the loading infusion rate may be desirable.
5.4 Arousability
Some patients receiving dexmedetomidine hydrochloride in 0.9% sodium chloride injection have been observed to be arousable and alert when stimulated. This alone should not be considered as evidence of lack of efficacy in the absence of other clinical signs and symptoms.
5.5 Withdrawal
Intensive Care Unit Sedation
With administration up to 7 days, regardless of dose, 12 (5%) dexmedetomidine hydrochloride in 0.9% sodium chloride injection adult subjects experienced at least 1 event related to withdrawal within the first 24 hours after discontinuing study drug and 7 (3%) dexmedetomidine hydrochloride in 0.9% sodium chloride injection adult subjects experienced at least 1 event 24 to 48 hours after end of study drug. The most common events were nausea, vomiting, and agitation.
In adult subjects, tachycardia and hypertension requiring intervention in the 48 hours following study drug discontinuation occurred at frequencies of <5%. If tachycardia and/or hypertension occurs after discontinuation of dexmedetomidine hydrochloride in 0.9% sodium chloride injection supportive therapy is indicated.
Procedural Sedation
In adult subjects, withdrawal symptoms were not seen after discontinuation of short term infusions of dexmedetomidine hydrochloride in 0.9% sodium chloride injection (<6 hours).
5.6 Tolerance and Tachyphylaxis
Use of dexmedetomidine beyond 24 hours has been associated with tolerance and tachyphylaxis and a dose-related increase in adverse reactions [see Adverse Reactions (6.1)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypotension, bradycardia and sinus arrest [see Warnings and Precautions (5.2)]
- Transient hypertension [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Most common treatment-emergent adverse reactions, occurring in greater than 2% of patients in both Intensive Care Unit and procedural sedation studies include hypotension, bradycardia and dry mouth.
Intensive Care Unit Sedation
Adverse reaction information is derived from the continuous infusion trials of dexmedetomidine for sedation in the Intensive Care Unit setting in which 1,007 adult patients received dexmedetomidine hydrochloride in 0.9% sodium chloride injection. The mean total dose was 7.4 mcg/kg (range: 0.8 to 84.1), mean dose per hour was 0.5 mcg/kg/hr (range: 0.1 to 6.0) and the mean duration of infusion of 15.9 hours (range: 0.2 to 157.2). The population was between 17 to 88 years of age, 43% ≥65 years of age, 77% male and 93% Caucasian. Treatment-emergent adverse reactions occurring at an incidence of >2% are provided in Table 2. The most frequent adverse reactions were hypotension; bradycardia and dry mouth [see Warnings and Precautions (5.2)].
Table 2: Adverse Reactions with an Incidence >2%-Adult Intensive Care Unit Sedation Population <24 hours*
| Adverse Event | All dexmedetomidine hydrochloride in 0.9% sodium chloride injection
(N = 1,007) (%) | Randomizeddexmedetomidine hydrochloride in 0.9% sodium chloride injection
(N = 798) (%) | Placebo
(N = 400)(%) | Propofol
(N = 188)(%) |
| Hypotension | 25% | 24% | 12% | 13% |
| Hypertension | 12% | 13% | 19% | 4% |
| Nausea | 9% | 9% | 9% | 11% |
| Bradycardia | 5% | 5% | 3% | 0 |
| Atrial Fibrillation | 4% | 5% | 3% | 7% |
| Pyrexia | 4% | 4% | 4% | 4% |
| Dry Mouth | 4% | 3% | 1% | 1% |
| Vomiting | 3% | 3% | 5% | 3% |
| Hypovolemia | 3% | 3% | 2% | 5% |
| Atelectasis | 3% | 3% | 3% | 6% |
| Pleural Effusion | 2% | 2% | 1% | 6% |
| Agitation | 2% | 2% | 3% | 1% |
| Tachycardia | 2% | 2% | 4% | 1% |
| Anemia | 2% | 2% | 2% | 2% |
| Hyperthermia | 2% | 2% | 3 % | 0 |
| Chills | 2% | 2% | 3 % | 2% |
| Hyperglycemia | 2% | 2% | 2% | 3% |
| Hypoxia | 2% | 2% | 2% | 3% |
| Post-procedural Hemorrhage | 2% | 2% | 3% | 4% |
| Pulmonary Edema | 1% | 1% | 1% | 3% |
| Hypocalcemia | 1% | 1% | 0 | 2% |
| Acidosis | 1% | 1% | 1% | 2% |
| Urine Output Decreased | 1% | 1% | 0 | 2% |
| Sinus Tachycardia | 1% | 1% | 1% | 2% |
| Ventricular Tachycardia | <1% | 1% | 1% | 5% |
| Wheezing | <1% | 1% | 0 | 2% |
| Edema Peripheral | <1% | 0 | 1% | 2% |
* 26 subjects in the all dexmedetomidine hydrochloride in 0.9% sodium chloride injection group and 10 subjects in the randomized dexmedetomidine hydrochloride in 0.9% sodium chloride injection group had exposure for greater than 24 hours.
Adverse reaction information was also derived from the placebo-controlled, continuous infusion trials of dexmedetomidine hydrochloride in 0.9% sodium chloride injection for sedation in the surgical intensive care unit setting in which 387 adult patients received dexmedetomidine hydrochloride in 0.9% sodium chloride injection for less than 24 hours. The most frequently observed treatment-emergent adverse events included hypotension, hypertension, nausea, bradycardia, fever, vomiting, hypoxia, tachycardia and anemia (see Table 3).
Table 3: Treatment-Emergent Adverse Events Occurring in >1% of All Dexmedetomidine-Treated Adult Patients in the Randomized Placebo-Controlled Continuous Infusion <24 Hours ICU Sedation Studies
| Adverse Event | Randomized Dexmedetomidine
(N = 387) | Placebo
(N = 379) |
| Hypotension | 28 % | 13% |
| Hypertension | 16 % | 18% |
| Nausea | 11% | 9% |
| Bradycardia | 7% | 3% |
| Fever | 5% | 4% |
| Vomiting | 4% | 6% |
| Atrial Fibrillation | 4% | 3% |
| Hypoxia | 4% | 4% |
| Tachycardia | 3% | 5% |
| Hemorrhage | 3% | 4% |
| Anemia | 3% | 2% |
| Dry Mouth | 3% | 1% |
| Rigors | 2% | 3% |
| Agitation | 2% | 3% |
| Hyperpyrexia | 2% | 3% |
| Pain | 2% | 2% |
| Hyperglycemia | 2% | 2% |
| Acidosis | 2% | 2% |
| Pleural Effusion | 2% | 1% |
| Oliguria | 2% | <1% |
| Thirst | 2% | <1% |
In a controlled clinical trial, dexmedetomidine hydrochloride in 0.9% sodium chloride injection was compared to midazolam for ICU sedation exceeding 24 hours duration in adult patients. Key treatment emergent adverse events occurring in dexmedetomidine or midazolam treated patients in the randomized active comparator continuous infusion long-term intensive care unit sedation study are provided in Table 4. The number (%) of subjects who had a dose-related increase in treatment-emergent adverse events by maintenance adjusted dose rate range in the dexmedetomidine hydrochloride in 0.9% sodium chloride injection group is provided in Table 5.
Table 4: Key Treatment-Emergent Adverse Events Occurring in Dexmedetomidine- or Midazolam-Treated Adult Patients in the Randomized Active Comparator Continuous Infusion Long-Term Intensive Care Unit Sedation Study
| Adverse Event | Dexmedetomidine (N = 244) | Midazolam (N = 122) |
| Hypotension1 | 56% | 56% |
| Hypotension Requiring Intervention | 28% | 27% |
| Bradycardia2 | 42% | 19% |
| Bradycardia Requiring Intervention | 5% | 1% |
| Systolic Hypertension3 | 28% | 42% |
| Tachycardia4 | 25% | 44% |
| Tachycardia Requiring Intervention | 10% | 10% |
| Diastolic Hypertension3 | 12% | 15% |
| Hypertension3 | 11% | 15% |
| Hypertension Requiring Intervention † | 19% | 30% |
| Hypokalemia | 9% | 13% |
| Pyrexia | 7% | 2% |
| Agitation | 7% | 6% |
| Hyperglycemia | 7% | 2% |
| Constipation | 6% | 6% |
| Hypoglycemia | 5% | 6% |
| Respiratory Failure | 5% | 3% |
| Renal Failure Acute | 2% | 1% |
| Acute Respiratory Distress Syndrome | 2% | 1% |
| Generalized Edema | 2% | 6% |
| Hypomagnesemia | 1% | 7% |
† Includes any type of hypertension
1 Hypotension was defined in absolute terms as Systolic blood pressure of <80 mmHg or Diastolic blood pressure of <50 mmHg or in relative terms as ≤30% lower than pre-study drug infusion value
2 Bradycardia was defined in absolute terms as <40 bpm or in relative terms as ≤30% lower than pre-study drug infusion value
3 Hypertension was defined in absolute terms as Systolic blood pressure >180 mmHg or Diastolic blood pressure of >100 mmHg or in relative terms as ≥30% higher than pre-study drug infusion value
4 Tachycardia was defined in absolute terms as >120 bpm or in relative terms as ≥30% greater than pre-study drug infusion value
The following adverse events occurred between 2 and 5% for dexmedetomidine hydrochloride in 0.9% sodium chloride injection and Midazolam, respectively: renal failure acute (2.5%, 0.8%), acute respiratory distress syndrome (2.5%, 0.8%), and respiratory failure (4.5%, 3.3%).
Table 5: Number (%) of Adult Subjects Who Had a Dose-Related Increase in Treatment Emergent Adverse Events by Maintenance Adjusted Dose Rate Range in the Dexmedetomidine hydrochloride in 0.9% sodium chloride injection Group
| Dexmedetomidine hydrochloride in 0.9% sodium chloride injection mcg/kg/hr | |||
| Adverse Event | ≤0.7*
(N = 95) | >0.7 to ≤1.1*
(N = 78) | >1.1*
(N = 71) |
| Constipation | 6% | 5% | 14% |
| Agitation | 5% | 8% | 14% |
| Anxiety | 5% | 5% | 9% |
| Edema Peripheral | 3% | 5% | 7% |
| Atrial Fibrillation | 2% | 4% | 9% |
| Respiratory Failure | 2% | 6% | 10% |
| Acute Respiratory Distress Syndrome | 1% | 3% | 9% |
* Average maintenance dose over the entire study drug administration
Procedural Sedation
Adverse reaction information is derived from the two trials for procedural sedation [see Clinical Studies (14.2)] in which 318 adult patients received dexmedetomidine hydrochloride in 0.9% sodium chloride injection. The mean total dose was 1.6 mcg/kg (range: 0.5 to 6.7), mean dose per hour was 1.3 mcg/kg/hr (range: 0.3 to 6.1) and the mean duration of infusion of 1.5 hours (range: 0.1 to 6.2). The population was between 18 to 93 years of age, ASA I-IV, 30% ≥65 years of age, 52% male and 61% Caucasian.
Treatment-emergent adverse reactions occurring at an incidence of >2% are provided in Table 6. The most frequent adverse reactions were hypotension, bradycardia, and dry mouth [see Warnings and Precautions (5.2)]. Pre-specified criteria for the vital signs to be reported as adverse reactions are footnoted below the table. The decrease in respiratory rate and hypoxia was similar between dexmedetomidine hydrochloride in 0.9% sodium chloride injection and comparator groups in both studies.
Table 6: Adverse Reactions with an Incidence > 2% - Procedural Sedation Population
| Adverse Event | Dexmedetomidine hydrochloride in
0.9% sodium chloride injection (N = 318) (%) | Placebo
(N = 113) (%) |
| Hypotension1 | 54% | 30% |
| Respiratory Depression2 | 37% | 32% |
| Bradycardia3 | 14% | 4% |
| Hypertension4 | 13% | 24% |
| Tachycardia5 | 5% | 17% |
| Nausea | 3% | 2% |
| Dry Mouth | 3% | 1% |
| Hypoxia6 | 2% | 3% |
| Bradypnea | 2% | 4% |
1 Hypotension was defined in absolute and relative terms as Systolic blood pressure of <80 mmHg or ≤30% lower than pre-study drug infusion value, or Diastolic blood pressure of <50 mmHg
2 Respiratory depression was defined in absolute and relative terms as respiratory rate (RR) <8 beats per minute or > 25% decrease from baseline
3 Bradycardia was defined in absolute and relative terms as <40 beats per minute or ≤30% lower than pre-study drug infusion value
4 Hypertension was defined in absolute and relative terms as Systolic blood pressure >180 mmHg or ≥30% higher than pre-study drug infusion value or Diastolic blood pressure of >100 mmHg
5 Tachycardia was defined in absolute and relative terms as >120 beats per minute or ≥30% greater than pre-study drug infusion value
6 Hypoxia was defined in absolute and relative terms as SpO2 <90% or 10% decrease from baseline
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of dexmedetomidine hydrochloride in 0.9% sodium chloride injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypotension and bradycardia were the most common adverse reactions associated with the use of dexmedetomidine hydrochloride in 0.9% sodium chloride injection during post-approval use of the drug.
Table 7: Adverse Reactions Experienced During Post-Approval Use of dexmedetomidine hydrochloride in 0.9% sodium chloride injection
| System Organ Class | Preferred Term |
| Blood and Lymphatic System Disorders | Anemia |
| Cardiac Disorders | Arrhythmia, atrial fibrillation, atrioventricular block, bradycardia, cardiac arrest, cardiac disorder, extrasystoles, myocardial infarction, supraventricular tachycardia, tachycardia, ventricular arrhythmia, ventricular tachycardia |
| Eye Disorders | Photopsia, visual impairment |
| Gastrointestinal Disorders | Abdominal pain, diarrhea, nausea, vomiting |
| General Disorders and Administration Site Conditions | Chills, hyperpyrexia, pain, pyrexia, thirst |
| Hepatobiliary Disorders | Hepatic function abnormal, hyperbilirubinemia |
| Investigations | Alanine aminotransferase increased, aspartate aminotransferase increased, blood alkaline phosphatase increased, blood urea increased, electrocardiogram T wave inversion, gammaglutamyltransferase increased, electrocardiogram QT prolonged |
| Metabolism and Nutrition Disorders | Acidosis, hyperkalemia, hypoglycemia, hypovolemia, hypernatremia |
| Nervous System Disorders | Convulsion, dizziness, headache, neuralgia, neuritis, speech disorder |
| Psychiatric Disorders | Agitation, confusional state, delirium, hallucination, illusion |
| Renal and Urinary Disorders | Oliguria, Polyuria |
| Respiratory, Thoracic and Mediastinal Disorders | Apnea, bronchospasm, dyspnea, hypercapnia, hypoventilation, hypoxia, pulmonary congestion, respiratory acidosis |
| Skin and Subcutaneous Tissue Disorders | Hyperhidrosis, pruritus, rash, utricaria |
| Surgical and Medical Procedures | Light anesthesia |
| Vascular Disorders | Blood pressure fluctuation, hemorrhage, hypertension, hypotension |
7 DRUG INTERACTIONS
7.1 Anesthetics, Sedatives, Hypnotics, Opioids
Co-administration of dexmedetomidine hydrochloride in 0.9% sodium chloride injection with anesthetics, sedatives, hypnotics, and opioids is likely to lead to an enhancement of effects. Specific studies have confirmed these effects with sevoflurane, isoflurane, propofol, alfentanil, and midazolam. No pharmacokinetic interactions between dexmedetomidine and isoflurane, propofol, alfentanil and midazolam have been demonstrated. However, due to possible pharmacodynamic interactions, when co-administered with dexmedetomidine, a reduction in dosage of dexmedetomidine hydrochloride in 0.9% sodium chloride injection or the concomitant anesthetic, sedative, hypnotic or opioid may be required.
7.2 Neuromuscular Blockers
In one study of 10 healthy adult volunteers, administration of dexmedetomidine hydrochloride in 0.9% sodium chloride injection for 45 minutes at a plasma concentration of one ng/mL resulted in no clinically meaningful increases in the magnitude of neuromuscular blockade associated with rocuronium administration.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies of dexmedetomidine hydrochloride in 0.9% sodium chloride injection use in pregnant women. In an in vitro human placenta study, placental transfer of dexmedetomidine occurred. In a study in the pregnant rat, placental transfer of dexmedetomidine was observed when radiolabeled dexmedetomidine was administered subcutaneously. Thus, fetal exposure should be expected in humans, and dexmedetomidine hydrochloride in 0.9% sodium chloride injection should be used during pregnancy only if the potential benefits justify the potential risk to the fetus.
Teratogenic effects were not observed in rats following subcutaneous administration of dexmedetomidine during the period of fetal organogenesis (from gestation day 5 to 16) with doses up to 200 mcg/kg (representing a dose approximately equal to the maximum recommended human intravenous dose based on body surface area) or in rabbits following intravenous administration of dexmedetomidine during the period of fetal organogenesis (from gestation day 6 to 18) with doses up to 96 mcg/kg (representing approximately half the human exposure at the maximum recommended dose based on plasma area under the time-curve comparison). However, fetal toxicity, as evidenced by increased post-implantation losses and reduced live pups, was observed in rats at a subcutaneous dose of 200 mcg/kg. The no-effect dose in rats was 20 mcg/kg (representing a dose less than the maximum recommended human intravenous dose based on a body surface area comparison). In another reproductive toxicity study when dexmedetomidine was administered subcutaneously to pregnant rats at 8 and 32 mcg/kg (representing a dose less than the maximum recommended human intravenous dose based on a body surface area comparison) from gestation day 16 through weaning, lower offspring weights were observed. Additionally, when offspring of the 32 mcg/kg group were allowed to mate, elevated fetal and embryocidal toxicity and delayed motor development was observed in second generation offspring.
8.2 Labor and Delivery
The safety of dexmedetomidine hydrochloride in 0.9% sodium chloride injection during labor and delivery has not been studied
8.3 Nursing Mothers
It is not known whether dexmedetomidine is excreted in human milk. Radio-labeled dexmedetomidine administered subcutaneously to lactating female rats was excreted in milk. Because many drugs are excreted in human milk, caution should be exercised when dexmedetomidine hydrochloride in 0.9% sodium chloride injection is administered to a nursing woman.
8.4 Pediatric Use
Safety and efficacy have not been established for Procedural or ICU Sedation in pediatric patients. One assessor-blinded trial in pediatric patients and two open label studies in neonates were conducted to assess efficacy for ICU sedation. These studies did not meet their primary efficacy endpoints and the safety data submitted were insufficient to fully characterize the safety profile of dexmedetomidine hydrochloride in 0.9% sodium chloride injection for this patient population The use of dexmedetomidine hydrochloride in 0.9% sodium chloride injection for procedural sedation in pediatric patients has not been evaluated.
8.5 Geriatric Use
Intensive Care Unit Sedation
A total of 729 patients in the clinical studies were 65 years of age and over. A total of 200 patients were 75 years of age and over. In patients greater than 65 years of age, a higher incidence of bradycardia and hypotension was observed following administration of dexmedetomidine hydrochloride in 0.9% sodium chloride injection [see Warnings and Precautions (5.2)]. Therefore, a dose reduction may be considered in patients over 65 years of age [see Dosage and Administration (2.2, 2.3) and Clinical Pharmacology (12.3)].
Procedural Sedation
A total of 131 patients in the clinical studies were 65 years of age and over. A total of 47 patients were 75 years of age and over. Hypotension occurred in a higher incidence in dexmedetomidine-treated patients 65 years or older (72%) and 75 years or older (74%) as compared to patients <65 years (47%). A reduced loading dose of 0.5 mcg/kg given over 10 minutes is recommended and a reduction in the maintenance infusion should be considered for patients greater than 65 years of age.
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
The dependence potential of dexmedetomidine hydrochloride in 0.9% sodium chloride injection has not been studied in humans. However, since studies in rodents and primates have demonstrated that dexmedetomidine exhibits pharmacologic actions similar to those of clonidine, it is possible that dexmedetomidine hydrochloride in 0.9% sodium chloride injection may produce a clonidine-like withdrawal syndrome upon abrupt discontinuation [see Warnings and Precautions (5.5)].
10 OVERDOSAGE
The tolerability of dexmedetomidine hydrochloride in 0.9% sodium chloride injection was studied in one study in which healthy adult subjects were administered doses at and above the recommended dose of 0.2 to 0.7 mcg/kg/hr. The maximum blood concentration achieved in this study was approximately 13 times the upper boundary of the therapeutic range. The most notable effects observed in two subjects who achieved the highest doses were first degree atrioventricular block and second degree heart block. No hemodynamic compromise was noted with the atrioventricular block and the heart block resolved spontaneously within one minute.
Five adult patients received an overdose of dexmedetomidine hydrochloride in 0.9% sodium chloride injection in the intensive care unit sedation studies. Two of these patients had no symptoms reported; one patient received a 2 mcg/kg loading dose over 10 minutes (twice the recommended loading dose) and one patient received a maintenance infusion of 0.8 mcg/kg/hr. Two other patients who received a 2 mcg/kg loading dose over 10 minutes, experienced bradycardia and/or hypotension. One patient who received a loading bolus dose of undiluted dexmedetomidine (19.4 mcg/kg), had cardiac arrest from which he was successfully resuscitated.
11 DESCRIPTION
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is a sterile, nonpyrogenic ready to use solution suitable for intravenous infusion. Dexmedetomidine hydrochloride, USP is the S-enantiomer of medetomidine and is chemically described as (+)-4-(S)-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole monohydrochloride. Dexmedetomidine hydrochloride, USP has a molecular weight of 236.7 and the empirical formula is C13H16N2•HCl and the structural formula is:
Dexmedetomidine hydrochloride, USP is a white or almost white powder that is freely soluble in water and has a pKa of 7.1. Its partition coefficient in-octanol: water at pH 7.4 is 2.89.
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is supplied as a clear, colorless, isotonic solution with a pH of 4.5 to 8.0. Each mL contains 4.72 mcg of dexmedetomidine hydrochloride, USP equivalent to 4 mcg (0.004 mg) of dexmedetomidine and 9 mg sodium chloride in water and is ready to be used. The solution is preservative-free and contains no additives or chemical stabilizers.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is a relatively selective alpha2-adrenergic agonist with sedative properties. Alpha2 selectivity is observed in animals following slow intravenous infusion of low and medium doses (10-300 mcg/kg). Both alpha1 and alpha2 activity is observed following slow intravenous infusion of high doses (≥1,000 mcg/kg) or with rapid intravenous administration.
12.2 Pharmacodynamics
In a study in healthy volunteers (N = 10), respiratory rate and oxygen saturation remained within normal limits and there was no evidence of respiratory depression when dexmedetomidine hydrochloride in 0.9% sodium chloride injection was administered by intravenous infusion at doses within the recommended dose range (0.2–0.7 mcg/kg/hr).
12.3 Pharmacokinetics
Following intravenous administration, dexmedetomidine exhibits the following pharmacokinetic parameters: a rapid distribution phase with a distribution half-life (t1/2) of approximately 6 minutes; a terminal elimination half-life (t1/2) of approximately 2 hours; and steady-state volume of distribution (Vss) of approximately 118 liters. Clearance is estimated to be approximately 39 L/h. The mean body weight associated with this clearance estimate was 72 kg.
Dexmedetomidine exhibits linear pharmacokinetics in the dosage range of 0.2 to 0.7 mcg/kg/hr when administered by intravenous infusion for up to 24 hours. Table 8 shows the main pharmacokinetic parameters when dexmedetomidine hydrochloride in 0.9% sodium chloride injection was infused (after appropriate loading doses) at maintenance infusion rates of 0.17 mcg/kg/hr (target plasma concentration of 0.3 ng/mL) for 12 and 24 hours, 0.33 mcg/kg/hr (target plasma concentration of 0.6 ng/mL) for 24 hours, and 0.70 mcg/kg/hr (target plasma concentration of 1.25 ng/mL) for 24 hours.
Table 8: Mean ± SD Pharmacokinetic Parameters
| Parameter | Loading Infusion (min)/Total Infusion Duration (hrs) | |||
| 10 min/12 hrs | 10 min/24 hrs | 10 min/24 hrs | 35 min/24 hrs | |
| Dexmedetomidine Target Plasma Concentration (ng/mL) and
Dose(mcg/kg/hr) |
||||
| 0.3/0.17 | 0.3/0.17 | 0.6/0.33 | 1.25/0.70 | |
| t1/2*, hour | 1.78 ± 0.30 | 2.22 ± 0.59 | 2.23 ± 0.21 | 2.50 ± 0.61 |
| CL, liter/hour | 46.3 ± 8.3 | 43.1 ± 6.5 | 35.3 ± 6.8 | 36.5 ± 7.5 |
| Vss, liter | 88.7 ± 22.9 | 102.4 ± 20.3 | 93.6 ± 17.0 | 99.6 ± 17.8 |
| Avg Css #, ng/mL | 0.27 ± 0.05 | 0.27 ± 0.05 | 0.67 ± 0.10 | 1.37 ± 0.20 |
Abbreviations: t1/2 = half life, CL = clearance, Vss = steady-state volume of distribution
* Presented as harmonic mean and pseudo standard deviation.
# Mean Css = Average steady-state concentration of dexmedetomidine. The mean Css was calculated based on post-dose sampling from 2.5 to 9 hours samples for 12 hour infusion and post-dose sampling from 2.5 to 18 hours for 24 hour infusions.
The loading doses for each of the above indicated groups were 0.5, 0.5, 1 and 2.2 mcg/kg, respectively.
Dexmedetomidine pharmacokinetic parameters after dexmedetomidine hydrochloride in 0.9% sodium chloride injection maintenance doses of 0.2 to 1.4 mcg/kg/hr for >24 hours were similar to the pharmacokinetic (PK) parameters after dexmedetomidine hydrochloride in 0.9% sodium chloride injection maintenance dosing for < 24 hours in other studies. The values for clearance (CL), volume of distribution (V), and t1/2 were 39.4 L/hr, 152 L, and 2.67 hours, respectively.
Distribution
The steady-state volume of distribution (Vss) of dexmedetomidine was approximately 118 liters. Dexmedetomidine protein binding was assessed in the plasma of normal healthy male and female subjects. The average protein binding was 94% and was constant across the different plasma concentrations tested. Protein binding was similar in males and females. The fraction of dexmedetomidine that was bound to plasma proteins was significantly decreased in subjects with hepatic impairment compared to healthy subjects.
The potential for protein binding displacement of dexmedetomidine by fentanyl, ketorolac, theophylline, digoxin and lidocaine was explored in vitro, and negligible changes in the plasma protein binding of dexmedetomidine were observed. The potential for protein binding displacement of phenytoin, warfarin, ibuprofen, propranolol, theophylline and digoxin by dexmedetomidine was explored in vitro and none of these compounds appeared to be significantly displaced by dexmedetomidine hydrochloride in 0.9% sodium chloride injection.
Elimination
Metabolism
Dexmedetomidine undergoes almost complete biotransformation with very little unchanged dexmedetomidine excreted in urine and feces. Biotransformation involves both direct glucuronidation as well as cytochrome P450 mediated metabolism. The major metabolic pathways of dexmedetomidine are: direct N-glucuronidation to inactive metabolites; aliphatic hydroxylation (mediated primarily by CYP2A6 with a minor role of CYP1A2, CYP2E1, CYP2D6 and CYP2C19) of dexmedetomidine to generate 3-hydroxy-dexmedetomidine, the glucuronide of 3-hydroxydexmedetomidine, and 3-carboxy-dexmedetomidine; and N-methylation of dexmedetomidine to generate 3-hydroxy N-methyl-dexmedetomidine, 3-carboxy N-methyl-dexmedetomidine, and dexmedetomidine-N-methyl O-glucuronide.
Excretion
The terminal elimination half-life (t1/2) of dexmedetomidine is approximately 2 hours and clearance is estimated to be approximately 39 L/h. A mass balance study demonstrated that after nine days an average of 95% of the radioactivity, following intravenous administration of radiolabeled dexmedetomidine, was recovered in the urine and 4% in the feces. No unchanged dexmedetomidine was detected in the urine. Approximately 85% of the radioactivity recovered in the urine was excreted within 24 hours after the infusion. Fractionation of the radioactivity excreted in urine demonstrated that products of N-glucuronidation accounted for approximately 34% of the cumulative urinary excretion. In addition, aliphatic hydroxylation of parent drug to form 3-hydroxy-dexmedetomidine, the glucuronide of 3-hydroxy-dexmedetomidine, and 3-carboxylic acid-dexmedetomidine together represented approximately 14% of the dose in urine. N-methylation of dexmedetomidine to form 3-hydroxy N-methyl dexmedetomidine, 3-carboxy N-methyl dexmedetomidine, and N-methyl O-glucuronide dexmedetomidine accounted for approximately 18% of the dose in urine. The N-Methyl metabolite itself was a minor circulating component and was undetected in urine. Approximately 28% of the urinary metabolites have not been identified.
Specific Populations
Male and Female Patients
There was no observed difference in dexmedetomidine pharmacokinetics due to gender.
Geriatric patients
The pharmacokinetic profile of dexmedetomidine was not altered by age. There were no differences in the pharmacokinetics of dexmedetomidine in young (18–40 years), middle age (41–65 years), and elderly (>65 years) subjects.
Patients with Hepatic Impairment
In subjects with varying degrees of hepatic impairment (Child-Pugh Class A, B, or C), clearance values for dexmedetomidine were lower than in healthy subjects. The mean clearance values for patients with mild, moderate, and severe hepatic impairment were 74%, 64% and 53% of those observed in the normal healthy subjects, respectively. Mean clearances for free drug were 59%, 51% and 32% of those observed in the normal healthy subjects, respectively.
Although dexmedetomidine hydrochloride in 0.9% sodium chloride injection is dosed to effect, it may be necessary to consider dose reduction in subjects with hepatic impairment [see Dosage and Administration (2.2), Warnings and Precautions (5.7)].
Patients with Renal Impairment
Dexmedetomidine pharmacokinetics (Cmax, Tmax, AUC, t1/2, CL, and Vss) were not significantly different in patients with severe renal impairment (creatinine clearance: <30 mL/min) compared to healthy subjects.
Drug Interaction studies
In vitro studies: In vitro studies in human liver microsomes demonstrated no evidence of cytochrome P450 mediated drug interactions that are likely to be of clinical relevance.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis
Animal carcinogenicity studies have not been performed with dexmedetomidine.
Mutagenesis
Dexmedetomidine was not mutagenic in vitro, in either the bacterial reverse mutation assay (E. coli and Salmonella typhimurium) or the mammalian cell forward mutation assay (mouse lymphoma). Dexmedetomidine was clastogenic in the in vitro human lymphocyte chromosome aberration test with, but not without, rat S9 metabolic activation. In contrast, dexmedetomidine was not clastogenic in the in vitro human lymphocyte chromosome aberration test with or without human S9 metabolic activation. Although dexmedetomidine was clastogenic in an in vivo mouse micronucleus test in NMRI mice, there was no evidence of clastogenicity in CD-1 mice.
Impairment of Fertility
Fertility in male or female rats was not affected after daily subcutaneous injections of dexmedetomidine at doses up to 54 mcg/kg (less than the maximum recommended human intravenous dose on a mcg/m2 basis) administered from 10 weeks prior to mating in males, and 3 weeks prior to mating and during mating in females.
13.2 Animal Pharmacology and/or Toxicology
There were no differences in the adrenocorticotropic hormone (ACTH)-stimulated cortisol response in dogs following a single dose of dexmedetomidine compared to saline control. However, after continuous subcutaneous infusions of dexmedetomidine at 3 mcg/kg/hr and 10 mcg/kg/hr for one week in dogs (exposures estimated to be within the clinical range), the ACTH-stimulated cortisol response was diminished by approximately 27% and 40%, respectively, compared to saline-treated control animals indicating a dose-dependent adrenal suppression.
14 CLINICAL STUDIES
The safety and efficacy of dexmedetomidine hydrochloride in 0.9% sodium chloride injection has been evaluated in four randomized, double-blind, placebo-controlled multicenter clinical trials in 1,185 adult patients.
14.1 Intensive Care Unit Sedation
Two randomized, double-blind, parallel-group, placebo-controlled multicenter clinical trials included 754 adult patients being treated in a surgical intensive care unit. All patients were initially intubated and received mechanical ventilation. These trials evaluated the sedative properties of dexmedetomidine hydrochloride in 0.9% sodium chloride injection by comparing the amount of rescue medication (midazolam in one trial and propofol in the second) required to achieve a specified level of sedation (using the standardized Ramsay Sedation Scale) between dexmedetomidine hydrochloride in 0.9% sodium chloride injection and placebo from onset of treatment to extubation or to a total treatment duration of 24 hours. The Ramsay Level of Sedation Scale is displayed in Table 9.
Table 9: Ramsay Level of Sedation Scale
| Clinical Score | Level of Sedation Achieved |
| 6 | Asleep, no response |
| 5 | Asleep, sluggish response to light glabellar tap or loud auditory stimulus |
| 4 | Asleep, but with brisk response to light glabellar tap or loud auditory stimulus |
| 3 | Patient responds to commands |
| 2 | Patient cooperative, oriented, and tranquil |
| 1 | Patient anxious, agitated, or restless |
In the first study, 175 adult patients were randomized to receive placebo and 178 to receive dexmedetomidine hydrochloride in 0.9% sodium chloride injection by intravenous infusion at a dose of 0.4 mcg/kg/hr (with allowed adjustment between 0.2 and 0.7 mcg/kg/hr) following an initial loading infusion of one mcg/kg intravenous over 10 minutes. The study drug infusion rate was adjusted to maintain a Ramsay sedation score of ≥3. Patients were allowed to receive “rescue” midazolam as needed to augment the study drug infusion. In addition, morphine sulfate was administered for pain as needed. The primary outcome measure for this study was the total amount of rescue medication (midazolam) needed to maintain sedation as specified while intubated. Patients randomized to placebo received significantly more midazolam than patients randomized to dexmedetomidine hydrochloride in 0.9% sodium chloride injection (see Table 10).
A second prospective primary analysis assessed the sedative effects of dexmedetomidine hydrochloride in 0.9% sodium chloride injection by comparing the percentage of patients who achieved a Ramsay sedation score of ≥3 during intubation without the use of additional rescue medication. A significantly greater percentage of patients in the dexmedetomidine hydrochloride group maintained a Ramsay sedation score of ≥3 without receiving any midazolam rescue compared to the placebo group (see Table 10).
Table 10: Midazolam Use as Rescue Medication During Intubation (ITT) Study One
| Placebo
(N = 175) | Dexmedetomidine hydrochloride in
0.9% sodium chloride injection (N = 178) | p-value | |
| Mean Total Dose (mg) of Midazolam
Standard deviation | 19 mg 53 mg | 5 mg 19 mg | 0.0011* |
| Categorized Midazolam Use | |||
| 0 mg | 43 (25%) | 108 (61%) | <0.001** |
| 0–4 mg | 34 (19%) | 36 (20%) | |
| >4 mg | 98 (56%) | 34 (19%) | |
ITT (intent-to-treat) population includes all randomized patients.
* ANOVA model with treatment center.
** Chi-square.
A prospective secondary analysis assessed the dose of morphine sulfate administered to patients in the dexmedetomidine hydrochloride in 0.9% sodium chloride injection and placebo groups. On average, dexmedetomidine hydrochloride in 0.9% sodium chloride injection-treated patients received less morphine sulfate for pain than placebo-treated patients (0.47 versus 0.83 mg/h). In addition, 44% (79 of 178 patients) of dexmedetomidine hydrochloride in 0.9% sodium chloride injection patients received no morphine sulfate for pain versus 19% (33 of 175 patients) in the placebo group.
In a second study, 198 adult patients were randomized to receive placebo and 203 to receive dexmedetomidine hydrochloride in 0.9% sodium chloride injection by intravenous infusion at a dose of 0.4 mcg/kg/hr (with allowed adjustment between 0.2 and 0.7 mcg/kg/hr) following an initial loading infusion of one mcg/kg intravenous over 10 minutes. The study drug infusion was adjusted to maintain a Ramsay sedation score of ≥3. Patients were allowed to receive “rescue” propofol as needed to augment the study drug infusion. In addition, morphine sulfate was administered as needed for pain. The primary outcome measure for this study was the total amount of rescue medication (propofol) needed to maintain sedation as specified while intubated.
Patients randomized to placebo received significantly more propofol than patients randomized to dexmedetomidine hydrochloride in 0.9% sodium chloride injection (see Table 11).
A significantly greater percentage of patients in the dexmedetomidine hydrochloride in 0.9% sodium chloride injection group compared to the placebo group maintained a Ramsay sedation score of ≥3 without receiving any propofol rescue (see Table 11).
Table 11: Propofol Use as Rescue Medication During Intubation (ITT) Study Two
| Placebo
(N = 198) | Dexmedetomidine hydrochloride in
0.9% sodium chloride injection (N = 203) | p-value | |
| Mean Total Dose (mg) of PropofolStandard deviation | 513 mg 782 mg | 72 mg 249 mg | <0.0001* |
| Categorized Propofol Use | |||
| 0 mg | 47 (24%) | 122 (60%) | <0.001** |
| 0–50 mg | 30 (15%) | 43 (21%) | |
| >50 mg | 121 (61%) | 38 (19%) | |
* ANOVA model with treatment center
** Chi-square
A prospective secondary analysis assessed the dose of morphine sulfate administered to patients in the dexmedetomidine hydrochloride in 0.9% sodium chloride injection and placebo groups. On average, dexmedetomidine hydrochloride in 0.9% sodium chloride injection-treated patients received less morphine sulfate for pain than placebo-treated patients (0.43 versus 0.89 mg/h). In addition, 41% (83 of 203 patients) of dexmedetomidine hydrochloride in 0.9% sodium chloride injection patients received no morphine sulfate for pain versus 15% (30 of 198 patients) in the placebo group.
In a controlled clinical trial, dexmedetomidine hydrochloride in 0.9% sodium chloride injection was compared to midazolam for ICU sedation exceeding 24 hours duration. Dexmedetomidine hydrochloride in 0.9% sodium chloride injection was not shown to be superior to midazolam for the primary efficacy endpoint, the percent of time patients were adequately sedated (81% versus 81%). In addition, administration of dexmedetomidine hydrochloride in 0.9% sodium chloride injection for longer than 24 hours was associated with tolerance, tachyphylaxis, and a dose-related increase in adverse events [see Adverse Reactions (6.1)].
14.2 Procedural Sedation
The safety and efficacy of dexmedetomidine hydrochloride in 0.9% sodium chloride injection for sedation of non-intubated patients prior to and/or during surgical and other procedures was evaluated in two randomized, double-blind, placebo-controlled multicenter clinical trials. Study 1 evaluated the sedative properties of dexmedetomidine in patients having a variety of elective surgeries/procedures performed under monitored anesthesia care. Study 2 evaluated dexmedetomidine hydrochloride in 0.9% sodium chloride injection in patients undergoing awake fiberoptic intubation prior to a surgical or diagnostic procedure.
In Study 1, the sedative properties of dexmedetomidine hydrochloride in 0.9% sodium chloride injection were evaluated by comparing the percent of patients not requiring rescue midazolam to achieve a specified level of sedation using the standardized Observer’s Assessment of Alertness/Sedation Scale (see Table 12).
Table 12: Observer’s Assessment of Alertness/Sedation
| Assessment Categories | ||||
| Responsiveness | Speech | Facial Expression | Eyes | Composite Score |
| Responds readily to name spoken in normal tone | Normal | Normal | Clear, no ptosis | 5 (alert) |
| Lethargic response to name spoken in normal tone | Mild slowing or thickening | Mild relaxation | Glazed or mild ptosis (less than half the eye) | 4 |
| Responds only after name is called loudly and/or repeatedly | Slurring or prominent slowing | Marked relaxation (slack jaw) | Glazed and marked ptosis (half the eye or more) | 3 |
| Responds only after mild prodding or shaking | Few recognizable words | – | – | 2 |
| Does not respond to mild prodding or shaking | – | – | – | 1 (deep sleep) |
Patients were randomized to receive a loading infusion of either dexmedetomidine hydrochloride in 0.9% sodium chloride injection 1 mcg/kg, dexmedetomidine hydrochloride in 0.9% sodium chloride injection 0.5 mcg/kg, or placebo (normal saline) given over 10 minutes and followed by a maintenance infusion started at 0.6 mcg/kg/hr. The maintenance infusion of study drug could be titrated from 0.2 mcg/kg/hr to 1 mcg/kg/hr to achieve the targeted sedation score (Observer’s Assessment of Alertness/Sedation Scale ≤4). Patients were allowed to receive rescue midazolam as needed to achieve and/or maintain an Observer’s Assessment of Alertness/Sedation Scale ≤4. After achieving the desired level of sedation, a local or regional anesthetic block was performed. Demographic characteristics were similar between the dexmedetomidine hydrochloride in 0.9% sodium chloride injection and comparator groups. Efficacy results showed that dexmedetomidine hydrochloride in 0.9% sodium chloride injection was more effective than the comparator group when used to sedate non-intubated patients requiring monitored anesthesia care during surgical and other procedures (see Table 13).
In Study 2, the sedative properties of dexmedetomidine hydrochloride in 0.9% sodium chloride injection were evaluated by comparing the percent of patients requiring rescue midazolam to achieve or maintain a specified level of sedation using the Ramsay Sedation Scale score ≥2 (see Table 9). Patients were randomized to receive a loading infusion of dexmedetomidine hydrochloride in 0.9% sodium chloride injection 1 mcg/kg or placebo (normal saline) given over 10 minutes and followed by a fixed maintenance infusion of 0.7 mcg/kg/hr. After achieving the desired level of sedation, topicalization of the airway occurred. Patients were allowed to receive rescue midazolam as needed to achieve and/or maintain a Ramsay Sedation Scale ≥2. Demographic characteristics were similar between the dexmedetomidine hydrochloride in 0.9% sodium chloride injection and comparator groups. For efficacy results see Table 13.
Table 13: Key Efficacy Results of Procedural Sedation Studies
| Study | Loading Infusion Treatment Arm | Number of Patients Enrolleda | % Not Requiring Midazolam Rescue | Confidenceb Interval on the Difference vs. Placebo | Mean (SD) Total Dose (mg) of
Rescue Midazolam Required | Confidenceb
Intervals of the Mean Rescue Dose |
| Study1 | Dexmedetomidine 0.5 mcg/kg | 134 | 40 | 37 (27, 48) | 1.4 (1.7) | -2.7 (-3.4, -2.0) |
| Dexmedetomidine 1 mcg/kg | 129 | 54 | 51 (40, 62) | 0.9 (1.5) | -3.1 (-3.8, -2.5) | |
| Placebo | 63 | 3 | – | 4.1 (3.0) | – | |
| Study2 | Dexmedetomidine 1 mcg/kg | 55 | 53 | 39 (20, 57) | 1.1 (1.5) | -1.8 (-2.7, -0.9) |
| Placebo | 50 | 14 | – | 2.9 (3.0) | – |
a Based on ITT population defined as all randomized and treated patients
b Normal approximation to the binomial with continuity correction
16 HOW SUPPLIED/STORAGE AND HANDLING
Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection
Dexmedetomidine hydrochloride in 0.9% sodium chloride Injection is a clear, colorless, sterile, nonpyrogenic ready to use solution suitable for intravenous infusion and available as 200 mcg/50 mL (4 mcg/mL) and 400 mcg/100 mL (4 mcg/mL) in 50 mL and 100 mL clear glass vials, respectively. The strength is based on the dexmedetomidine base. Containers are intended for single-dose only. Discard unused portion.
| NDC No. | Container | Size | Package Factor |
| 43598-976-58 | Single-dose vial | 50 mL | 10 Vials per Carton |
| 43598-975-58 | Single-dose vial | 100 mL | 10 Vials per Carton |
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is indicated for short-term intravenous sedation. Dosage must be individualized and titrated to the desired clinical effect. Blood pressure, heart rate and oxygen levels will be monitored both continuously during the infusion of dexmedetomidine hydrochloride in 0.9% sodium chloride injection and as clinically appropriate after discontinuation.
- When dexmedetomidine hydrochloride in 0.9% sodium chloride injection is infused for more than 6 hours, patients should be informed to report nervousness, agitation, and headaches that may occur for up to 48 hours.
- Additionally, patients should be informed to report symptoms that may occur within 48 hours after the administration of dexmedetomidine hydrochloride in 0.9% sodium chloride injection such as: weakness, confusion, excessive sweating, weight loss, abdominal pain, salt cravings, diarrhea, constipation, dizziness or light-headedness.
Rx only
Distributor:
Dr. Reddy’s Laboratories Inc.,
Princeton, NJ 08540
Made in India
Issued: 0520
INGREDIENTS AND APPEARANCE
| DEXMEDETOMIDINE HYDROCHLORIDE
dexmedetomidine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DEXMEDETOMIDINE HYDROCHLORIDE
dexmedetomidine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Dr.Reddy's Laboratories Inc (802315887) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GLAND PHARMA LIMITED | 918601238 | manufacture(43598-976, 43598-975) , analysis(43598-976, 43598-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GLAND PHARMA LIMITED | 858971074 | analysis(43598-976, 43598-975) , manufacture(43598-976, 43598-975) | |