Search by Drug Name or NDC
NDC 44118-0700-10 Plexion 98; 48 mg/g; mg/g Details
Plexion 98; 48 mg/g; mg/g
Plexion is a TOPICAL LIQUID in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Eckson Labs, LLC. The primary component is SULFACETAMIDE SODIUM; SULFUR.
Product Information
| NDC | 44118-0700 |
|---|---|
| Product ID | 44118-700_d91769aa-ca47-567f-e053-2a95a90a664b |
| Associated GPIs | 90059903200917 |
| GCN Sequence Number | 072168 |
| GCN Sequence Number Description | sulfacetamide sodium/sulfur CLEANSER 9.8%-4.8% TOPICAL |
| HIC3 | Q5S |
| HIC3 Description | TOPICAL SULFONAMIDES |
| GCN | 36287 |
| HICL Sequence Number | 003344 |
| HICL Sequence Number Description | SULFACETAMIDE SODIUM/SULFUR |
| Brand/Generic | Brand |
| Proprietary Name | Plexion |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | SULFACETAMIDE SODIUM, SULFUR |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | LIQUID |
| Route | TOPICAL |
| Active Ingredient Strength | 98; 48 |
| Active Ingredient Units | mg/g; mg/g |
| Substance Name | SULFACETAMIDE SODIUM; SULFUR |
| Labeler Name | Eckson Labs, LLC |
| Pharmaceutical Class | Sulfonamide Antibacterial [EPC], Sulfonamides [CS] |
| DEA Schedule | n/a |
| Marketing Category | UNAPPROVED DRUG OTHER |
| Application Number | n/a |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 44118-0700-10 (44118070010)
| NDC Package Code | 44118-700-10 |
|---|---|
| Billing NDC | 44118070010 |
| Package | 285 g in 1 BOTTLE, PLASTIC (44118-700-10) |
| Marketing Start Date | 2022-02-28 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL d91769aa-ca46-567f-e053-2a95a90a664b Details
DESCRIPTION:
Each gram contains 98 mg of sodium sulfacetamide and 48 mg of colloidal sulfur in a vehicle consisting of: benzyl alcohol, cetyl alcohol, fragrance, glyceryl stearate (and) PEG-100 stearate, magnesium aluminum silicate, phenoxyethanol, propylene glycol, purified water, sodium lauryl sulfate, sodium lithium magnesium silicate, sodium thiosulfate, stearyl alcohol and xanthan gum.
Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Sodium sulfacetamide is C 8H 9N 2NaO 3S·H 2O with molecular weight of 254.24. Chemically, sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
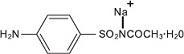
Sodium sulfacetamide is an odorless, white, crystalline powder with a bitter taste. It is freely soluble in water, sparingly soluble in alcohol, while practically insoluble in benzene, in chloroform and in ether.
CLINICAL PHARMACOLOGY:
Sodium sulfacetamide exerts a bacteriostatic effect against sulfonamide sensitive Gram-positive and Gram-negative microorganisms commonly isolated from secondary cutaneous pyogenic infections. It acts by restricting the synthesis of folic acid required by bacteria for growth, by its competition with para-aminobenzoic acid. There is no clinical data available on the degree and rate of systemic absorption of this product when applied to the skin or scalp. However, significant absorption of sodium sulfacetamide through the skin has been reported.
The following in vitro data is available but the clinical significance is unknown. Organisms that show susceptibility to sodium sulfacetamide are: Streptococci, Staphylococci, E. coli, Klebsiella pneumoniae, Pseudomonas pyocyanea, Salmonella species, Proteus vulgaris, Nocardia and Actinomyces.
The exact mode of action of sulfur in the treatment of acne is unknown, but it has been reported that it inhibits the growth of Propionibacterium acnes and the formation of free fatty acids.
INDICATIONS AND USAGE:
CONTRAINDICATIONS:
WARNINGS:
Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitive individuals. Stevens-Johnson syndrome also has been reported following the use of sodium sulfacetamide topically. Cases of drug-induced systemic lupus erythematosus from topical sulfacetamide also have been reported. In one of these cases, there was a fatal outcome. KEEP OUT OF THE REACH OF CHILDREN.
PRECAUTIONS: FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
General: Nonsusceptible organisms, including fungi, may proliferate with the use of this preparation.
Although rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. If the use of this product produces signs of hypersensitivity or other untoward reactions, discontinue use of the preparation. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved. Systemic absorption of topical sulfonamides is greater following application to large, infected, abraded, denuded or severely burned areas. Under these circumstances, any of the adverse effects produced by the systemic administration of these agents could potentially occur, and appropriate observations and laboratory determinations should be performed. The object of this therapy is to achieve desquamation without irritation, but sodium sulfacetamide and sulfur can cause reddening and scaling of the epidermis. These side effects are not unusual in the treatment of acne vulgaris, but patients should be cautioned about the possibility.
Information for Patients: Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or elsewhere. The use of this product also should be discontinued promptly and the physician notified if any arthritis, fever or sores in the mouth develop. Avoid contact with eyes, lips and mucous membranes.
Drug Interactions: This product is incompatible with silver preparations.
Carcinogenesis, Mutagenesis and Impairment of Fertility: Long-term animal studies for carcinogenic potential have not been performed on this product to date. Studies on reproduction and fertility also have not been performed. Chromosomal nondisjunction has been reported in the yeast, Saccharomyces cerevisiae, following application of sodium sulfacetamide. The significance of this finding to the topical use of sodium sulfacetamide in the human is unknown.
Pregnancy:Category C. Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this product is administered to a nursing woman.
Pediatric Use: Safety and effectiveness in children under the age of 12 years have not been established.
ADVERSE REACTIONS:
Reports of irritation and hypersensitivity to sodium sulfacetamide are uncommon. The following adverse reactions, reported after administration of sterile ophthalmic sodium sulfacetamide, are noteworthy: instances of Stevens-Johnson syndrome and instances of local hypersensitivity which progressed to a syndrome resembling systemic lupus erythematosus; in one case a fatal outcome was reported (see WARNINGS).
OVERDOSAGE:
The oral LD 50 of sulfacetamide in mice is 16.5 g/kg. In the event of overdosage, emergency treatment should be started immediately.
Manifestations: Overdosage may cause nausea and vomiting. Large oral overdosage may cause hematuria, crystalluria and renal shutdown due to the precipitation of sulfa crystals in the renal tubules and the urinary tract. For treatment, contact your local Poison Control Center or your doctor.
DOSAGE AND ADMINISTRATION:
Wash affected areas once or twice daily, or as directed by your physician. Wet skin and liberally apply to areas to be cleansed. Massage gently into skin for 10 to 20 seconds, working into a full lather, rinse thoroughly and pat dry. If skin dryness occurs, it may be controlled by rinsing product off sooner or using less frequently.
STORAGE:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
NOTICE: Protect from freezing and excessive heat. The product may tend to darken slightly on storage. Slight discoloration does not impair the efficacy or safety of the product. Keep bottle tightly closed.
Occasionally, a slight discoloration of fabric may occur when an excessive amount of the product is used and comes in contact with white fabrics. This discoloration, however, presents no problem, as it is readily removed by ordinary laundering without bleaches.
HOW SUPPLIED:
INGREDIENTS AND APPEARANCE
| PLEXION
sulfacetamide sodium, sulfur liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Eckson Labs, LLC (078435242) |