Search by Drug Name or NDC
NDC 50804-0126-05 Goodsense Extra Strength Cold and Hot Medicated 50 mg/1 Details
Goodsense Extra Strength Cold and Hot Medicated 50 mg/1
Goodsense Extra Strength Cold and Hot Medicated is a TOPICAL PATCH in the HUMAN OTC DRUG category. It is labeled and distributed by Geiss, Destin and Dunn, Inc.. The primary component is MENTHOL.
Product Information
| NDC | 50804-0126 |
|---|---|
| Product ID | 50804-126_dfee9135-b092-6d82-e053-2995a90a181a |
| Associated GPIs | 90070060005930 |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Goodsense Extra Strength Cold and Hot Medicated |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Menthol |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | PATCH |
| Route | TOPICAL |
| Active Ingredient Strength | 50 |
| Active Ingredient Units | mg/1 |
| Substance Name | MENTHOL |
| Labeler Name | Geiss, Destin and Dunn, Inc. |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part348 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
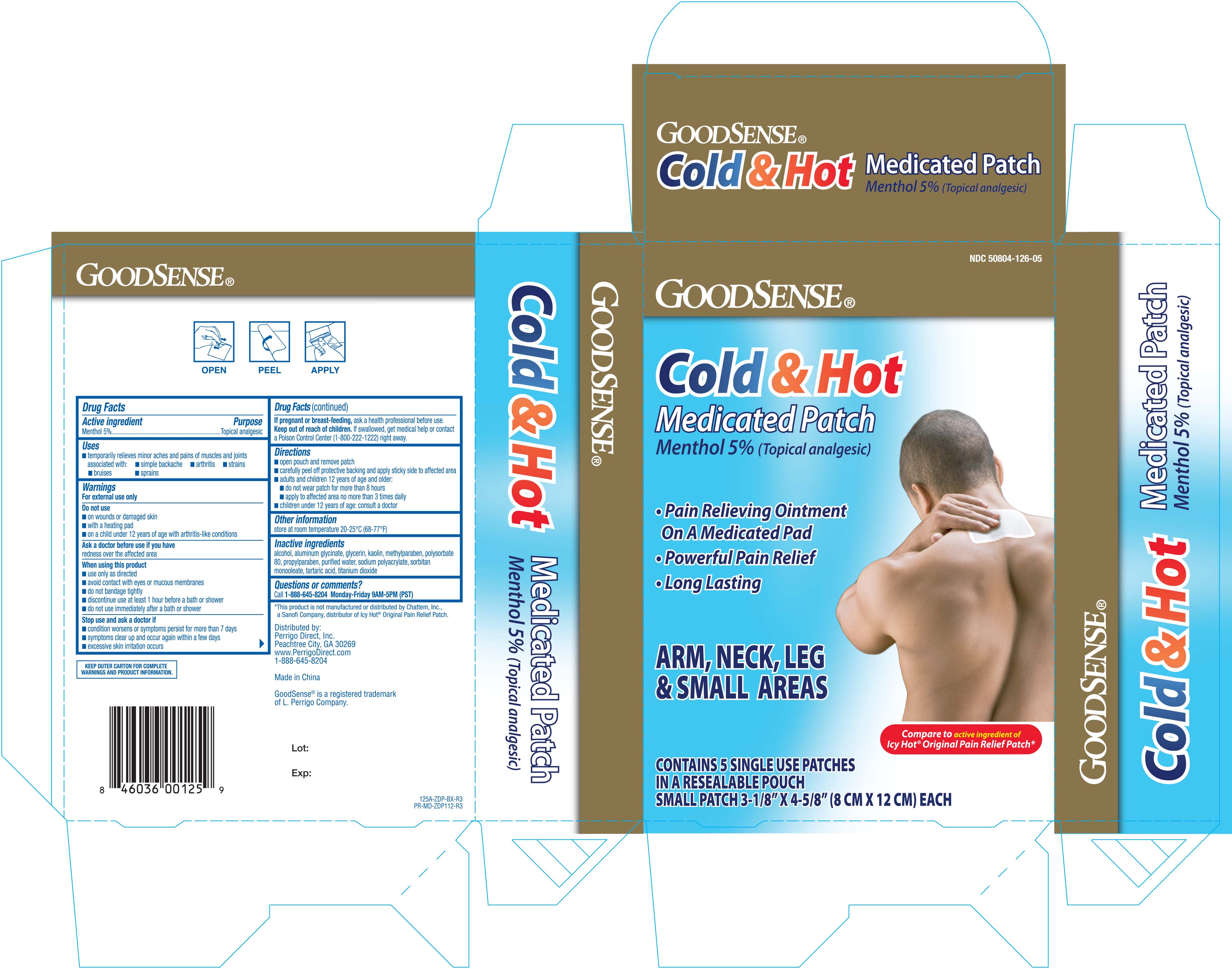
NDC 50804-0126-05 (50804012605)
| NDC Package Code | 50804-126-05 |
|---|---|
| Billing NDC | 50804012605 |
| Package | 1 POUCH in 1 CARTON (50804-126-05) / 5 PATCH in 1 POUCH |
| Marketing Start Date | 2018-12-26 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 7585d111-775f-4fb6-a485-28cd99d88852 Details
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
Directions
- open pouch and remove patch
- carefully peel off protective backing and apply sticky side to affected area
- adults and children 12 years of age or older
- do not wear patch for more than 8 hours
- apply to affected area no more than 3 times daily
- children under 12 years of age: consult a doctor
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
INGREDIENTS AND APPEARANCE
| GOODSENSE EXTRA STRENGTH COLD AND HOT MEDICATED
menthol patch |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Geiss, Destin and Dunn, Inc. (076059836) |
Revised: 5/2022
Document Id: dfee9135-b092-6d82-e053-2995a90a181a
Set id: 7585d111-775f-4fb6-a485-28cd99d88852
Version: 9
Effective Time: 20220526