Search by Drug Name or NDC
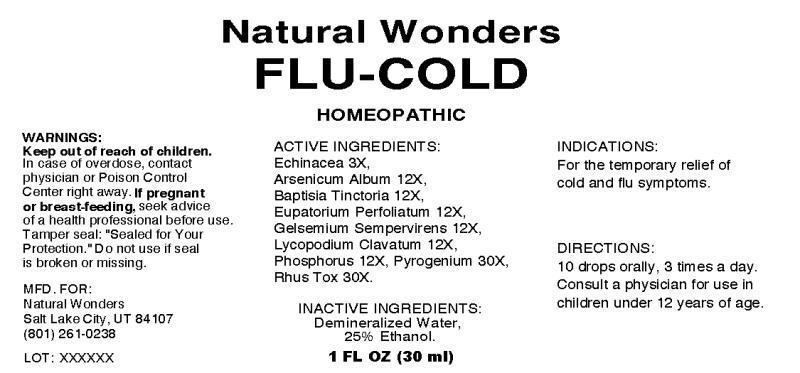
NDC 54146-0001-01 Flu-Cold 12; 12; 3; 12; 12; 12; 12; 30; 30 [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL Details
Flu-Cold 12; 12; 3; 12; 12; 12; 12; 30; 30 [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL
Flu-Cold is a ORAL LIQUID in the HUMAN OTC DRUG category. It is labeled and distributed by Natural Wonders. The primary component is ARSENIC TRIOXIDE; BAPTISIA TINCTORIA ROOT; ECHINACEA ANGUSTIFOLIA; EUPATORIUM PERFOLIATUM FLOWERING TOP; GELSEMIUM SEMPERVIRENS ROOT; LYCOPODIUM CLAVATUM SPORE; PHOSPHORUS; RANCID BEEF; TOXICODENDRON PUBESCENS LEAF.
Product Information
| NDC | 54146-0001 |
|---|---|
| Product ID | 54146-0001_a78d4bb4-b188-426e-ac73-e1aa95480fa4 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Flu-Cold |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Echinacea, Arsenicum Album, Baptisia Tinctoria, Eupatorium Perfoliatum, Gelsemium Sempervirens, Lycopodium Clavatum, Phosphorus, Pyrogenium, Rhus Toxicodendron |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | LIQUID |
| Route | ORAL |
| Active Ingredient Strength | 12; 12; 3; 12; 12; 12; 12; 30; 30 |
| Active Ingredient Units | [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL |
| Substance Name | ARSENIC TRIOXIDE; BAPTISIA TINCTORIA ROOT; ECHINACEA ANGUSTIFOLIA; EUPATORIUM PERFOLIATUM FLOWERING TOP; GELSEMIUM SEMPERVIRENS ROOT; LYCOPODIUM CLAVATUM SPORE; PHOSPHORUS; RANCID BEEF; TOXICODENDRON PUBESCENS LEAF |
| Labeler Name | Natural Wonders |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | UNAPPROVED HOMEOPATHIC |
| Application Number | n/a |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 54146-0001-01 (54146000101)
| NDC Package Code | 54146-0001-1 |
|---|---|
| Billing NDC | 54146000101 |
| Package | 30 mL in 1 BOTTLE, DROPPER (54146-0001-1) |
| Marketing Start Date | 2013-04-30 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 46f674d1-8a50-4ebe-a0db-d539b75d9129 Details
ACTIVE INGREDIENTS:
WARNINGS:
DIRECTIONS:
KEEP OUT OF REACH OF CHILDREN:
INGREDIENTS AND APPEARANCE
| FLU-COLD
echinacea, arsenicum album, baptisia tinctoria, eupatorium perfoliatum, gelsemium sempervirens, lycopodium clavatum, phosphorus, pyrogenium, rhus toxicodendron liquid |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Natural Wonders (153675293) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(54146-0001) , api manufacture(54146-0001) , label(54146-0001) , pack(54146-0001) | |
Revised: 2/2020
Document Id: a78d4bb4-b188-426e-ac73-e1aa95480fa4
Set id: 46f674d1-8a50-4ebe-a0db-d539b75d9129
Version: 5
Effective Time: 20200218