Search by Drug Name or NDC
NDC 57896-0339-05 Ear Wax Removal Drops 65 mg/mL Details
Ear Wax Removal Drops 65 mg/mL
Ear Wax Removal Drops is a TOPICAL SOLUTION/ DROPS in the HUMAN OTC DRUG category. It is labeled and distributed by GeriCare Pharmaceutical Corp. The primary component is CARBAMIDE PEROXIDE.
Product Information
| NDC | 57896-0339 |
|---|---|
| Product ID | 57896-339_fc3b137e-909a-4170-9a3b-83feaa333cd9 |
| Associated GPIs | 87400030002010 |
| GCN Sequence Number | 008120 |
| GCN Sequence Number Description | carbamide peroxide DROPS 6.5 % OTIC (EAR) |
| HIC3 | Q8R |
| HIC3 Description | EAR PREPARATIONS,EAR WAX REMOVERS |
| GCN | 34401 |
| HICL Sequence Number | 004272 |
| HICL Sequence Number Description | CARBAMIDE PEROXIDE |
| Brand/Generic | Brand |
| Proprietary Name | Ear Wax Removal Drops |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Carbamide Peroxide - 6.5% |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | SOLUTION/ DROPS |
| Route | TOPICAL |
| Active Ingredient Strength | 65 |
| Active Ingredient Units | mg/mL |
| Substance Name | CARBAMIDE PEROXIDE |
| Labeler Name | GeriCare Pharmaceutical Corp |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part344 |
| Listing Certified Through | 2022-12-31 |
Package
Package Images

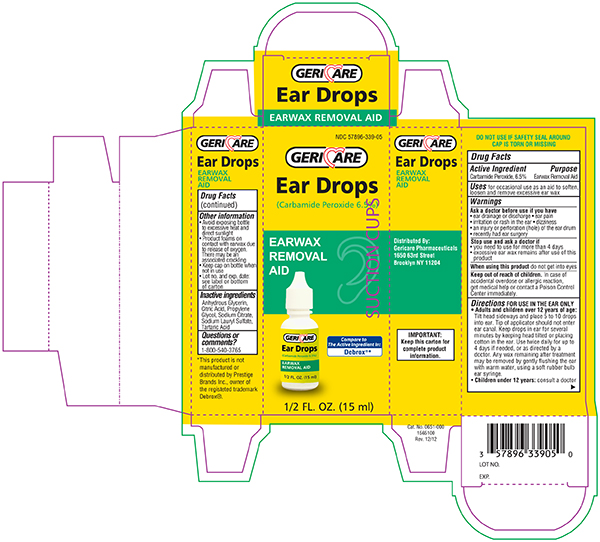
NDC 57896-0339-05 (57896033905)
| NDC Package Code | 57896-339-05 |
|---|---|
| Billing NDC | 57896033905 |
| Package | 1 BOTTLE, DROPPER in 1 CARTON (57896-339-05) / 15 mL in 1 BOTTLE, DROPPER |
| Marketing Start Date | 2014-05-27 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 239702af-cf93-43ee-b1af-8e53642395c1 Details
Warnings
When using this product
Keep out of the reach of children
Directions FOR USE IN THE EAR ONLY
- Adults and children over 12 years of age:
- Tilt head sideways and place 5 to 10 drops into ear.
- Tip of applicator should not enter ear canal.
- Keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear.
- Use twice daily for up to 4 days if needed, or as directed by a doctor.
- Any earwax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe.
- When the ear canal is irrigated, the tip of the ear syringe should not obstruct the flow of water leaving the ear canal.
- Children under 12 years: consult a doctor.
Other information
Inactive ingredients
Principal Display Panel Bottle Label 0.5 FL OZ
Principal Display Panel - Carton label 0.5 FL OZ
INGREDIENTS AND APPEARANCE
| EAR WAX REMOVAL DROPS
carbamide peroxide - 6.5% solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GeriCare Pharmaceutical Corp (611196254) |
| Registrant - Sheffield Pharmaceuticals LLC (151177797) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sheffield Pharmaceuticals LLC | 151177797 | manufacture(57896-339) | |
Revised: 12/2017
Document Id: fc3b137e-909a-4170-9a3b-83feaa333cd9
Set id: 239702af-cf93-43ee-b1af-8e53642395c1
Version: 1
Effective Time: 20171228