Search by Drug Name or NDC
NDC 58790-0001-31 TheraTears Lubricant 2.5 mg/mL Details
TheraTears Lubricant 2.5 mg/mL
TheraTears Lubricant is a OPHTHALMIC SOLUTION/ DROPS in the HUMAN OTC DRUG category. It is labeled and distributed by MEDTECH PRODUCTS INC. The primary component is CARBOXYMETHYLCELLULOSE SODIUM.
Product Information
| NDC | 58790-0001 |
|---|---|
| Product ID | 58790-001_a536ead7-0286-4223-873b-4129c70a2175 |
| Associated GPIs | 86200010102010 |
| GCN Sequence Number | 024192 |
| GCN Sequence Number Description | carboxymethylcellulose sodium DROPS 0.25 % OPHTHALMIC |
| HIC3 | Q6T |
| HIC3 Description | ARTIFICIAL TEARS |
| GCN | 37382 |
| HICL Sequence Number | 003843 |
| HICL Sequence Number Description | CARBOXYMETHYLCELLULOSE SODIUM |
| Brand/Generic | Brand |
| Proprietary Name | TheraTears Lubricant |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | carboxymethylcellulose sodium |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | SOLUTION/ DROPS |
| Route | OPHTHALMIC |
| Active Ingredient Strength | 2.5 |
| Active Ingredient Units | mg/mL |
| Substance Name | CARBOXYMETHYLCELLULOSE SODIUM |
| Labeler Name | MEDTECH PRODUCTS INC |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part349 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
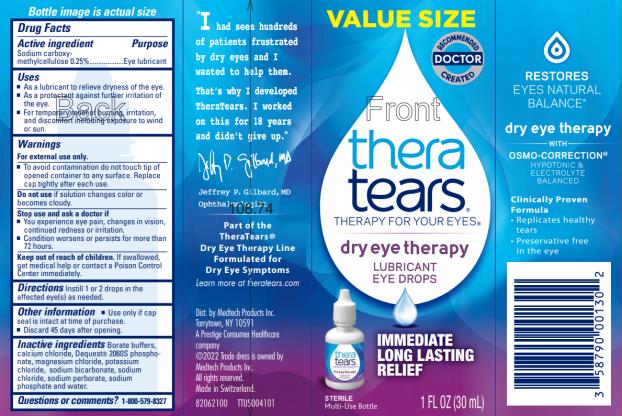
NDC 58790-0001-31 (58790000131)
| NDC Package Code | 58790-001-31 |
|---|---|
| Billing NDC | 58790000131 |
| Package | 2 BOTTLE, DROPPER in 1 CARTON (58790-001-31) / 30 mL in 1 BOTTLE, DROPPER |
| Marketing Start Date | 2020-12-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 661d8764-8ae6-4229-9485-867553ee8e16 Details
Uses
Warnings
Inactive ingredients
Principal Display Panel Text for Carton Label:
INGREDIENTS AND APPEARANCE
| THERATEARS LUBRICANT
carboxymethylcellulose sodium solution/ drops |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - MEDTECH PRODUCTS INC (114707784) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn AG | 482198285 | analysis(58790-001) , label(58790-001) , manufacture(58790-001) , pack(58790-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn Operating Company LLC (dba Akorn) | 117696790 | label(58790-001) , pack(58790-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn Operating Company LLC (dba Akorn) | 117696832 | analysis(58790-001) , manufacture(58790-001) , sterilize(58790-001) | |
Revised: 3/2022
Document Id: a536ead7-0286-4223-873b-4129c70a2175
Set id: 661d8764-8ae6-4229-9485-867553ee8e16
Version: 8
Effective Time: 20220308