Search by Drug Name or NDC
NDC 58809-0222-24 DAYCLEAR ALLERGY RELIEF CHEWABLES 25; 25 mg/1; mg/1 Details
DAYCLEAR ALLERGY RELIEF CHEWABLES 25; 25 mg/1; mg/1
DAYCLEAR ALLERGY RELIEF CHEWABLES is a ORAL TABLET, CHEWABLE in the HUMAN OTC DRUG category. It is labeled and distributed by GM Pharmaceuticals, INC. The primary component is CHLOPHEDIANOL HYDROCHLORIDE; PYRILAMINE MALEATE.
Product Information
| NDC | 58809-0222 |
|---|---|
| Product ID | 58809-222_d5907515-133d-20e6-e053-2995a90ad7f3 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | DAYCLEAR ALLERGY RELIEF CHEWABLES |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | CHLOPHEDIANOL HYDROCHLORIDE, Pyrilamine Maleate |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, CHEWABLE |
| Route | ORAL |
| Active Ingredient Strength | 25; 25 |
| Active Ingredient Units | mg/1; mg/1 |
| Substance Name | CHLOPHEDIANOL HYDROCHLORIDE; PYRILAMINE MALEATE |
| Labeler Name | GM Pharmaceuticals, INC |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
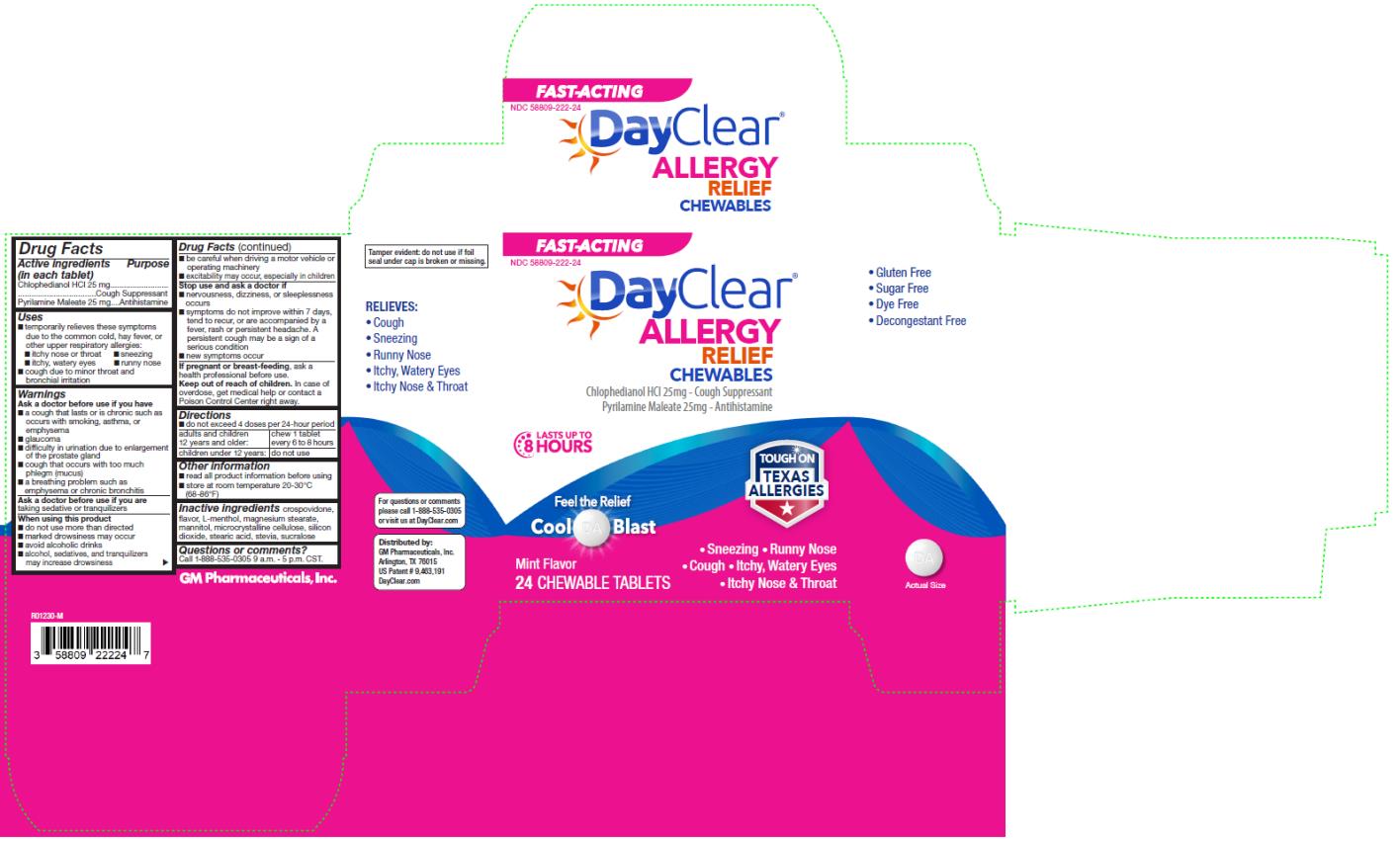
NDC 58809-0222-24 (58809022224)
| NDC Package Code | 58809-222-24 |
|---|---|
| Billing NDC | 58809022224 |
| Package | 1 BOTTLE in 1 CARTON (58809-222-24) / 24 TABLET, CHEWABLE in 1 BOTTLE |
| Marketing Start Date | 2020-01-02 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 4c600c0a-4254-4fe6-8c4c-99095c5412f2 Details
Uses
Warnings
Ask a doctor before use if you have
■ a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
■ glaucoma
■ difficulty in urination due to enlargement of the prostate gland
■ cough that occurs with too much phlegm (mucus)
■ a breathing problem such as emphysema or chronic bronchitis
When using this product
■ do not use more than directed
■ marked drowsiness may occur
■ avoid alcoholic drinks
■ alcohol, sedatives, and tranquilizers may increase drowsiness
■ be careful when driving a motor vehicle or operating machinery
■ excitability may occur, especially in children
Directions
Other information
Inactive ingredients
INGREDIENTS AND APPEARANCE
| DAYCLEAR ALLERGY RELIEF CHEWABLES
chlophedianol hydrochloride, pyrilamine maleate tablet, chewable |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - GM Pharmaceuticals, INC (793000860) |