Search by Drug Name or NDC
NDC 59651-0444-01 Valganciclovir 50 mg/mL Details
Valganciclovir 50 mg/mL
Valganciclovir is a ORAL FOR SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Aurobindo Pharma Limited. The primary component is VALGANCICLOVIR HYDROCHLORIDE.
Product Information
| NDC | 59651-0444 |
|---|---|
| Product ID | 59651-444_1e08a87f-624a-4993-a773-8c660c27b6af |
| Associated GPIs | |
| GCN Sequence Number | 064506 |
| GCN Sequence Number Description | valganciclovir HCl SOLN RECON 50 MG/ML ORAL |
| HIC3 | W5A |
| HIC3 Description | ANTIVIRALS, GENERAL |
| GCN | 14453 |
| HICL Sequence Number | 022033 |
| HICL Sequence Number Description | VALGANCICLOVIR HCL |
| Brand/Generic | Generic |
| Proprietary Name | Valganciclovir |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Valganciclovir hydrochloride powder, |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | FOR SOLUTION |
| Route | ORAL |
| Active Ingredient Strength | 50 |
| Active Ingredient Units | mg/mL |
| Substance Name | VALGANCICLOVIR HYDROCHLORIDE |
| Labeler Name | Aurobindo Pharma Limited |
| Pharmaceutical Class | Cytomegalovirus Nucleoside Analog DNA Polymerase Inhibitor [EPC], DNA Polymerase Inhibitors [MoA], Nucleoside Analog Antiviral [EPC], Nucleoside Analog [EXT] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA215124 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 59651-0444-01 (59651044401)
| NDC Package Code | 59651-444-01 |
|---|---|
| Billing NDC | 59651044401 |
| Package | 1 BOTTLE, GLASS in 1 CARTON (59651-444-01) / 100 mL in 1 BOTTLE, GLASS |
| Marketing Start Date | 2022-11-17 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 1e08a87f-624a-4993-a773-8c660c27b6af Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
VALGANCICLOVIR for oral solution
Initial U.S. Approval: 2001
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
See full prescribing information for complete boxed warning.
- Hematologic Toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, and bone marrow failure including aplastic anemia have been reported in patients treated with valganciclovir hydrochloride. (5.1)
- Impairment of Fertility: Based on animal data and limited human data, valganciclovir hydrochloride may cause temporary or permanent inhibition of spermatogenesis in males and suppression of fertility in females. (5.3)
- Fetal Toxicity: Based on animal data, valganciclovir hydrochloride has the potential to cause birth defects in humans. (5.4)
- Mutagenesis and Carcinogenesis: Based on animal data, valganciclovir hydrochloride has the potential to cause cancers in humans. (5.5)
INDICATIONS AND USAGE
Valganciclovir hydrochloride is a deoxynucleoside analogue cytomegalovirus (CMV) DNA polymerase inhibitor indicated for:
Adult Patients (1.1)
- Treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS).
- Prevention of CMV disease in kidney, heart, and kidney-pancreas transplant patients at high risk.
Pediatric Patients (1.2)
- Prevention of CMV disease in kidney and heart transplant patients at high risk.
DOSAGE AND ADMINISTRATION
| Adult Dosage (2.2)
|
|
| Treatment of CMV retinitis | Induction: 900 mg (two 450 mg tablets) twice a day for 21 days Maintenance: 900 mg (two 450 mg tablets) once a day |
| Prevention of CMV disease in heart or kidney-pancreas transplant patients | 900 mg (two 450 mg tablets) once a day within 10 days of transplantation until 100 days post-transplantation |
| Prevention of CMV disease in kidney transplant patients | 900 mg (two 450 mg tablets) once a day within 10 days of transplantation until 200 days post-transplantation |
| Pediatric Dosage (2.3)
|
|
| Prevention of CMV disease in kidney transplant patients 4 months to 16 years of age | Dose once a day within 10 days of transplantation until 200 days post-transplantation according to dosage algorithm (note the calculation of creatinine clearance using a modified Schwartz formula in children) |
| Prevention of CMV disease in heart transplant patients 1 month to 16 years of age | Dose once a day within 10 days of transplantation until 100 days post-transplantation according to dosage algorithm (note the calculation of creatinine clearance using a modified Schwartz formula in children) |
- Valganciclovir for oral solution and tablets should be taken with food. (2.1, 12.3)
- Valganciclovir tablets should not be broken or crushed. (2.6)
- Adult patients should use valganciclovir tablets, not valganciclovir for oral solution. (2.1)
- Adults with renal impairment: Adjust dose based on creatinine clearance. For adult patients receiving hemodialysis a dose recommendation cannot be given. (2.5, 8.6, 12.3)
DOSAGE FORMS AND STRENGTHS
- Oral Solution: 50 mg per mL. (3)
CONTRAINDICATIONS
Hypersensitivity to valganciclovir or ganciclovir. (4)
WARNINGS AND PRECAUTIONS
- Acute renal failure: Acute renal failure may occur in elderly patients (with or without reduced renal function), patients who receive concomitant nephrotoxic drugs, or inadequately hydrated patients. Use with caution in elderly patients or those taking nephrotoxic drugs, reduce dosage in patients with renal impairment, and monitor renal function. (2.5, 5.2, 8.5, 8.6)
ADVERSE REACTIONS
- Adult patients: Most common adverse reactions and laboratory abnormalities (reported in at least one indication by greater than or equal to 20% of patients) are diarrhea, pyrexia, fatigue, nausea, tremor, neutropenia, anemia, leukopenia, thrombocytopenia, headache, insomnia, urinary tract infection, and vomiting. (6.1)
- Pediatric patients: Most common adverse reactions and laboratory abnormalities (reported in greater than or equal to 20% of pediatric solid organ transplant recipients) are diarrhea, pyrexia, upper respiratory tract infection, urinary tract infection, vomiting, neutropenia, leukopenia, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Imipenem-cilastatin: Seizures were reported in patients receiving ganciclovir and imipenem-cilastatin. Concomitant use is not recommended unless the potential benefits outweigh the risks. (7)
- Cyclosporine or amphotericin B: When coadministered with valganciclovir, the risk of nephrotoxicity may be increased. Monitor renal function. (5.2, 7)
- Mycophenolate mofetil (MMF): When coadministered with valganciclovir, the risk of hematological and renal toxicity may be increased. Monitor for ganciclovir and MMF toxicity. (7)
- Other drugs associated with myelosuppression or nephrotoxicity: Due to potential for increased toxicity, consider for concomitant use with valganciclovir only if the potential benefits are judged to outweigh the risks. (7)
- Didanosine: Ganciclovir coadministered with didanosine may increase didanosine levels. Monitor for didanosine toxicity (e.g., pancreatitis). (7)
- Probenecid: May increase ganciclovir levels. Monitor for evidence of ganciclovir toxicity. (7)
USE IN SPECIFIC POPULATIONS
- Lactation: Breastfeeding is not recommended with use of valganciclovir hydrochloride. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
1 INDICATIONS AND USAGE
1.1 Adult Patients
1.2 Pediatric Patients
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Recommended Dosage in Adult Patients with Normal Renal Function
2.3 Recommended Dosage in Pediatric Patients
2.4 Preparation of Valganciclovir for Oral Solution
2.5 Dosage Recommendation for Adult Patients with Renal Impairment
2.6 Handling and Disposal
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic Toxicity
5.2 Acute Renal Failure
5.3 Impairment of Fertility
5.4 Fetal Toxicity
5.5 Mutagenesis and Carcinogenesis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Patients
14.2 Pediatric Patients
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
- Hematologic Toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, and bone marrow failure including aplastic anemia have been reported in patients treated with valganciclovir hydrochloride [see Warnings and Precautions (5.1)].
- Impairment of Fertility: Based on animal data and limited human data, valganciclovir hydrochloride may cause temporary or permanent inhibition of spermatogenesis in males and suppression of fertility in females [see Warnings and Precautions (5.3)].
- Fetal Toxicity: Based on animal data, valganciclovir hydrochloride has the potential to cause birth defects in humans [see Warnings and Precautions (5.4)].
- Mutagenesis and Carcinogenesis: Based on animal data, valganciclovir hydrochloride has the potential to cause cancers in humans [see Warnings and Precautions (5.5)].
1 INDICATIONS AND USAGE
1.1 Adult Patients
Treatment of Cytomegalovirus (CMV) Retinitis: Valganciclovir tablets are indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS) [see Clinical Studies (14.1)].
Prevention of CMV Disease: Valganciclovir tablets are indicated for the prevention of CMV disease in kidney, heart, and kidney-pancreas transplant patients at high risk (Donor CMV seropositive/Recipient CMV seronegative [D+/R-]) [see Clinical Studies (14.1)].
1.2 Pediatric Patients
Prevention of CMV Disease: Valganciclovir for oral solution is indicated for the prevention of CMV disease in kidney transplant patients (4 months to 16 years of age) and heart transplant patients (1 month to 16 years of age) at high risk [see Clinical Studies (14.2)].
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
- Adult patients should use valganciclovir tablets, not valganciclovir for oral solution.
- Valganciclovir for oral solution and tablets should be taken with food [see Clinical Pharmacology (12.3)].
- Valganciclovir for oral solution (50 mg/mL) must be prepared by the pharmacist prior to dispensing to the patient [see Dosage and Administration (2.4)].
2.2 Recommended Dosage in Adult Patients with Normal Renal Function
For dosage recommendations in adult patients with renal impairment [see Dosage and Administration (2.5)].
Treatment of CMV Retinitis:
- Induction: The recommended dosage is 900 mg (two 450 mg tablets) taken orally twice a day for 21 days.
- Maintenance: Following induction treatment, or in adult patients with inactive CMV retinitis, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day.
Prevention of CMV Disease:
- For adult patients who have received a heart or kidney-pancreas transplant, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day starting within 10 days of transplantation until 100 days post-transplantation.
- For adult patients who have received a kidney transplant, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day starting within 10 days of transplantation until 200 days post-transplantation.
2.3 Recommended Dosage in Pediatric Patients
Prevention of CMV Disease in Pediatric Kidney Transplant Patients: For pediatric kidney transplant patients 4 months to 16 years of age, the recommended once daily mg dose (7 x BSA x CrCl) should start within 10 days of post-transplantation until 200 days post-transplantation.
Prevention of CMV Disease in Pediatric Heart Transplant Patients: For pediatric heart transplant patients 1 month to 16 years of age, the recommended once daily mg dose (7 x BSA x CrCl) should start within 10 days of transplantation until 100 days post-transplantation.
The recommended once daily dosage of valganciclovir for oral solution is based on body surface area (BSA) and creatinine clearance (CrCl) derived from a modified Schwartz formula, and is calculated using the equation below:
Pediatric Dose (mg) = 7 x BSA x CrCl (calculated using a modified Schwartz formula). If the calculated Schwartz creatinine clearance exceeds 150 mL/min/1.73 m2, then a maximum value of 150 mL/min/1.73 m2 should be used in the equation. The k values used in the modified Schwartz formula are based on pediatric patient age, as shown in Table 1.

| *The k values provided are based on the Jaffe method of measuring serum creatinine, and may require correction when enzymatic methods are used1. | |
| k value
| Pediatric Patient Age
|
| 0.33 | Infants less than 1 year of age with low birth weight for gestational age |
| 0.45 | Infants less than 1 year of age with birth weight appropriate for gestational age |
| 0.45 | Children aged 1 to less than 2 years |
| 0.55 | Boys aged 2 to less than 13 years Girls aged 2 to less than 16 years |
| 0.7 | Boys aged 13 to 16 years |
Monitor serum creatinine levels regularly and consider changes in height and body weight and adapt the dose as appropriate during prophylaxis period.
All calculated doses should be rounded to the nearest 25 mg increment for the actual deliverable dose. The oral dispenser is graduated in 0.5 mL increments. A 50 mg dose is equivalent to 1 mL. If the calculated dose exceeds 900 mg, a maximum dose of 900 mg should be administered. Valganciclovir for oral solution is the preferred formulation since it provides the ability to administer a dose calculated according to the formula above; however, valganciclovir tablets may be used if the calculated doses are within 10% of available tablet strength (450 mg). For example, if the calculated dose is between 405 mg and 495 mg, one 450 mg tablet may be taken. Before prescribing valganciclovir tablets, pediatric patients should be assessed for the ability to swallow tablets.
2.4 Preparation of Valganciclovir for Oral Solution
Wearing disposable gloves is recommended during reconstitution and when wiping the outer surface of the bottle/cap and the table after reconstitution. Prior to dispensing to the patient, valganciclovir for oral solution must be prepared by the pharmacist as follows [see How Supplied/Storage and Handling (16)]:
- Measure 91 mL of purified water in a graduated cylinder.
- Shake the valganciclovir for oral solution bottle to loosen the powder. Remove the child resistant bottle cap and add approximately half the total amount of water for constitution to the bottle and shake the closed bottle well for about 1 minute. Add the remainder of water and shake the closed bottle well for about 1 minute. This prepared solution contains 50 mg of valganciclovir free base per 1 mL.
- Remove the child resistant bottle cap and push the bottle adapter into the neck of the bottle.
- Close bottle with child resistant bottle cap tightly. This will assure the proper seating of the bottle adapter in the bottle and child resistant status of the cap.
- Store constituted oral solution under refrigeration at 2ºC to 8ºC (36ºF to 46ºF) for no longer than 49 days. Do not freeze.
- Write the discard date of the constituted oral solution on the bottle label.
The patient package insert, which includes the dosing instructions for patients, and 2 oral dispensers should be dispensed to the patient [see Patient Counseling Information (17)].
2.5 Dosage Recommendation for Adult Patients with Renal Impairment
Serum creatinine levels or estimated creatinine clearance should be monitored regularly during treatment. Dosage recommendations for adult patients with reduced renal function are provided in Table 2. For adult patients on hemodialysis (CrCl less than 10 mL/min), a dose recommendation for valganciclovir tablets cannot be given [see Use in Specific Populations (8.5, 8.6), Clinical Pharmacology (12.3)].
Table 2 Dosage Recommendations for Adult Patients with Impaired Renal Function
| *An estimated creatinine clearance in adults is calculated from serum creatinine by the following formulas: | ||
| Valganciclovir 450 mg Tablets
|
||
| CrCl* (mL/min)
| Induction Dose
| Maintenance/
Prevention Dose |
| ≥ 60 | 900 mg twice daily | 900 mg once daily |
| 40 to 59 | 450 mg twice daily | 450 mg once daily |
| 25 to 39 | 450 mg once daily | 450 mg every 2 days |
| 10 to 24 | 450 mg every 2 days | 450 mg twice weekly |
| < 10 (on hemodialysis) | not recommended | not recommended |
(140 – age [years]) x (body weight [kg])
For males = ——————————————————
(72) x (serum creatinine [mg/dL])
For females = 0.85 x male value
Dosing in pediatric patients with renal impairment can be done using the recommended equations because CrCl is a component in the calculation [see Dosage and Administration (2.3)].
2.6 Handling and Disposal
Caution should be exercised in the handling of valganciclovir tablets and valganciclovir for oral solution. Tablets should not be broken or crushed. Because valganciclovir is considered a potential teratogen and carcinogen in humans, caution should be observed in handling broken tablets, the powder for oral solution, and the constituted oral solution [see Warnings and Precautions (5.4, 5.5)]. Avoid direct contact with broken or crushed tablets, the powder for oral solution, and the constituted oral solution with skin or mucous membranes. If such contact occurs, wash thoroughly with soap and water, and rinse eyes thoroughly with plain water.
Handle and dispose valganciclovir for oral solution according to guidelines for antineoplastic drugs because ganciclovir shares some of the properties of antitumor agents (i.e., carcinogenicity and mutagenicity).2
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
Valganciclovir for oral solution is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction (e.g., anaphylaxis) to valganciclovir, ganciclovir, or any component of the formulation [see Adverse Reactions (6.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic Toxicity
Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, and bone marrow failure including aplastic anemia have been reported in patients treated with valganciclovir hydrochloride or ganciclovir. Valganciclovir hydrochloride should be avoided if the absolute neutrophil count is less than 500 cells/µL, the platelet count is less than 25,000/µL, or the hemoglobin is less than 8 g/dL. Valganciclovir hydrochloride should also be used with caution in patients with pre-existing cytopenias and in patients receiving myelosuppressive drugs or irradiation. Cytopenia may occur at any time during treatment and may worsen with continued dosing. Cell counts usually begin to recover within 3 to 7 days after discontinuing drug. In patients with severe leukopenia, neutropenia, anemia and/or thrombocytopenia, treatment with hematopoietic growth factors may be considered.
Due to the frequency of neutropenia, anemia, and thrombocytopenia in patients receiving valganciclovir hydrochloride [see Adverse Reactions (6.1)], complete blood counts with differential and platelet counts should be performed frequently, especially in infants, in patients with renal impairment, and in patients in whom ganciclovir or other nucleoside analogues have previously resulted in leukopenia, or in whom neutrophil counts are less than 1000 cells/µL at the beginning of treatment. Increased monitoring for cytopenias may be warranted if therapy with oral ganciclovir is changed to valganciclovir hydrochloride because of increased plasma concentrations of ganciclovir after valganciclovir hydrochloride administration [see Clinical Pharmacology (12.3)].
5.2 Acute Renal Failure
Acute renal failure may occur in:
- Elderly patients with or without reduced renal function. Caution should be exercised when administering valganciclovir hydrochloride to geriatric patients, and dosage reduction is recommended for those with impaired renal function [see Dosage and Administration (2.5), Use in Specific Populations (8.5, 8.6)].
- Patients receiving potential nephrotoxic drugs. Caution should be exercised when administering valganciclovir hydrochloride to patients receiving potential nephrotoxic drugs.
- Patients without adequate hydration. Adequate hydration should be maintained for all patients.
5.3 Impairment of Fertility
Based on animal data and limited human data, valganciclovir hydrochloride at the recommended human doses may cause temporary or permanent inhibition of spermatogenesis in males, and may cause suppression of fertility in females. Advise patients that fertility may be impaired with use of valganciclovir hydrochloride [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
5.4 Fetal Toxicity
Ganciclovir may cause fetal toxicity when administered to pregnant women based on findings in animal studies. When given to pregnant rabbits at dosages resulting in 2 times the human exposure (based on AUC), ganciclovir caused malformations in multiple organs of the fetuses. Maternal and fetal toxicity were also observed in pregnant mice and rabbits. Therefore, valganciclovir hydrochloride has the potential to cause birth defects. Pregnancy should be avoided in female patients taking valganciclovir hydrochloride and in females with male partners taking valganciclovir hydrochloride. Females of reproductive potential should be advised to use effective contraception during treatment and for at least 30 days following treatment with valganciclovir hydrochloride because of the potential risk to the fetus. Similarly, males should be advised to use condoms during and for at least 90 days following treatment with valganciclovir hydrochloride [see Dosage and Administration (2.6), Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
5.5 Mutagenesis and Carcinogenesis
Animal data indicate that ganciclovir is mutagenic and carcinogenic. Valganciclovir hydrochloride should therefore be considered a potential carcinogen in humans [see Dosage and Administration (2.6), Nonclinical Toxicology (13.1)].
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Hematologic Toxicity [see Warnings and Precautions (5.1)].
- Acute Renal Failure [see Warnings and Precautions (5.2)].
- Impairment of Fertility [see Warnings and Precautions (5.3)].
- Fetal Toxicity [see Warnings and Precautions (5.4)].
- Mutagenesis and Carcinogenesis [see Warnings and Precautions (5.5)].
The most common adverse reactions and laboratory abnormalities reported in at least one indication by greater than or equal to 20% of adult patients treated with valganciclovir tablets are diarrhea, pyrexia, fatigue, nausea, tremor, neutropenia, anemia, leukopenia, thrombocytopenia, headache, insomnia, urinary tract infection, and vomiting. The most common reported adverse reactions and laboratory abnormalities reported in greater than or equal to 20% of pediatric solid organ transplant recipients treated with valganciclovir for oral solution or tablets are diarrhea, pyrexia, upper respiratory tract infection, urinary tract infection, vomiting, neutropenia, leukopenia, and headache.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
Valganciclovir, a prodrug of ganciclovir, is rapidly converted to ganciclovir after oral administration. Adverse reactions known to be associated with ganciclovir usage can therefore be expected to occur with valganciclovir hydrochloride.
Adverse Reactions in Adults:
Treatment of CMV Retinitis in AIDS Patients: In a clinical study for the treatment of CMV retinitis in HIV-infected patients, the adverse reactions reported by patients receiving valganciclovir tablets (n=79) or intravenous ganciclovir (n=79) for 28 days of randomized therapy (21 days induction dose and 7 days maintenance dose), respectively, included diarrhea (16%, 10%), nausea (8%, 14%), and headache (9%, 5%). The incidence of adverse reactions was similar between the group who received valganciclovir tablets and the group who received intravenous ganciclovir. The frequencies of neutropenia (ANC less than 500/µL) were 11% for patients receiving valganciclovir tablets compared with 13% for patients receiving intravenous ganciclovir. Anemia (Hgb less than 8 g/dL) occurred in 8% of patients in each group. Other laboratory abnormalities occurred with similar frequencies in the two groups.
Adverse reactions and laboratory abnormalities are available for 370 patients who received maintenance therapy with valganciclovir tablets 900 mg once daily in two open-label clinical trials. Approximately 252 (68%) of these patients received valganciclovir tablets for more than nine months (maximum duration was 36 months). Table 3 and Table 4 show pooled selected adverse reactions and abnormal laboratory values from these patients.
Table 3 Pooled Selected Adverse Reactions Reported in greater than or equal to 5% of Patients who Received Valganciclovir Tablets Maintenance Therapy for CMV Retinitis
| Patients with CMV Retinitis
|
|
| Adverse Reactions according to Body
System | Valganciclovir Tablets
(N=370) % |
| Gastrointestinal system
Diarrhea Nausea Vomiting Abdominal pain | 41 30 21 15 |
| General disorders and administrative site conditions
Pyrexia | 31 |
| Nervous system disorders
Headache Insomnia Neuropathy peripheral Paresthesia | 22 16 9 8 |
| Eye disorders
Retinal detachment |
15 |
Table 4 Pooled Selected Laboratory Abnormalities Reported in Patients Who Received Valganciclovir Tablets Maintenance Therapy for the Treatment of CMV Retinitis
| Patients with CMV Retinitis
|
|
| Laboratory Abnormalities
| Valganciclovir Tablets (N=370) % |
| Neutropenia: ANC/µL < 500 500 to < 750 750 to < 1000 | 19 17 17 |
| Anemia: Hemoglobin g/dL < 6.5 6.5 to < 8.0 8.0 to < 9.5 | 7 13 16 |
| Thrombocytopenia: Platelets/µL < 25000 25000 to < 50000 50000 to < 100000 | 4 6 22 |
| Serum Creatinine: mg/dL > 2.5 > 1.5 to 2.5 | 3 12 |
Prevention of CMV Disease in Solid Organ Transplant Patients: Table 5 shows selected adverse reactions regardless of severity with an incidence of greater than or equal to 5% from a clinical trial (up to 28 days after study treatment) where heart, kidney, kidney-pancreas and liver transplant patients received valganciclovir tablets (N=244) or oral ganciclovir (N=126) until Day 100 post-transplant. The majority of the adverse reactions were of mild or moderate intensity.
Table 5 Percentage of Selected Grades 1 to 4 Adverse Reactions Reported in greater than or equal to 5% of Adult Patients From a Study of Solid Organ Transplant Patients
| Adverse Reactions
| Valganciclovir Tablets
(N=244) % | Oral Ganciclovir
(N=126) % |
| Gastrointestinal disorders
| | |
| Diarrhea | 30 | 29 |
| Nausea | 23 | 23 |
| Vomiting | 16 | 14 |
| Nervous system disorders
| | |
| Tremors | 28 | 25 |
| Headache | 22 | 27 |
| Insomnia | 20 | 16 |
| General disorders and administration site conditions
| | |
| Pyrexia | 13 | 14 |
Table 6 shows selected adverse reactions regardless of severity with an incidence of greater than or equal to 5% from another clinical trial where kidney transplant patients received either valganciclovir once daily starting within 10 days post-transplant until Day 100 post-transplant followed by 100 days of placebo or valganciclovir once daily until Day 200 post-transplant. The overall safety profile of valganciclovir did not change with the extension of prophylaxis until Day 200 post-transplant in high risk kidney transplant patients.
Table 6 Percentage of Selected Grades 1 to 4 Adverse Reactions Reported in greater than or equal to 5% of Adult Patients from a Study of Kidney Transplant Patients
| Adverse Reactions
| Valganciclovir Tablets
Day 100 Post-transplant (N=164) % | Valganciclovir Tablets
Day 200 Post-transplant (N=156) % |
| Gastrointestinal disorders
| | |
| Diarrhea | 26 | 31 |
| Nausea | 11 | 11 |
| Vomiting | 3 | 6 |
| Nervous system disorders
| | |
| Tremors | 12 | 17 |
| Headache | 10 | 6 |
| Insomnia | 7 | 6 |
| General disorders and administration site conditions
| | |
| Pyrexia | 12 | 9 |
Table 7 and Table 8 show selected laboratory abnormalities reported with valganciclovir tablets in two trials in solid organ transplant patients.
Table 7 Selected Laboratory Abnormalities Reported in a Study of Adult Solid Organ Transplant Patients*
| *Laboratory abnormalities are those reported by investigators. | ||
| Laboratory Abnormalities
| Valganciclovir Tablets
(N=244) % | Ganciclovir Capsules (N=126) % |
| Neutropenia: ANC/µL < 500 500 to < 750 750 to < 1000 | 5 3 5 | 3 2 2 |
| Anemia: Hemoglobin g/dL < 6.5 6.5 to < 8.0 8.0 to < 9.5 | 1 5 31 | 2 7 25 |
| Thrombocytopenia: Platelets/µL < 25000 25000 to < 50000 50000 to < 100000 | 0 1 18 | 2 3 21 |
| Serum Creatinine: mg/dL > 2.5 > 1.5 to 2.5 | 14 45 | 21 47 |
Table 8 Selected Laboratory Abnormalities Reported in a Study of Adult Kidney Transplant Patients*
| *Laboratory abnormalities are those reported by investigators. | ||
| Laboratory Abnormalities
| Valganciclovir Tablets
Day 100 Post-transplant (N=164) % | Valganciclovir Tablets
Day 200 Post-transplant (N=156) % |
| Neutropenia: ANC/µL < 500 500 to < 750 750 to < 1000 | 9 6 7 | 10 6 5 |
| Anemia: Hemoglobin g/dL < 6.5 6.5 to < 8.0 8.0 to < 9.5 | 0 5 17 | 1 1 15 |
| Thrombocytopenia: Platelets/µL < 25000 25000 to < 50000 50000 to < 100000 | 0 1 7 | 0 0 3 |
| Serum Creatinine: mg/dL > 2.5 > 1.5 to 2.5 | 17 50 | 14 48 |
Other adverse drug reactions from valganciclovir hydrochloride in clinical trials in CMV retinitis and solid organ transplant patients
Other adverse drug reactions with valganciclovir hydrochloride in clinical trials in either patients with CMV retinitis or solid organ transplant patients that occurred in at least 5% of patients are listed below.
Eye disorders: retinal detachment, eye pain
Gastrointestinal disorders: dyspepsia, constipation, abdominal distention, mouth ulceration General disorders and administration site conditions: fatigue, pain, malaise, asthenia, chills, peripheral edema
Hepatobiliary disorders: hepatic function abnormal
Infections and infestations: candida infections including oral candidiasis, upper respiratory tract infection, influenza, urinary tract infection, pharyngitis/nasopharyngitis, postoperative wound infection
Injury, poisoning, and procedural complications: postoperative complications, wound secretion Metabolic and nutrition disorders: decreased appetite, hyperkalemia, hypophosphatemia, weight decreased
Musculoskeletal and connective tissue disorders: back pain, myalgia, arthralgia, muscle spasms Nervous system disorders: insomnia, neuropathy peripheral, dizziness
Psychiatric disorders: depression, anxiety
Renal and urinary disorders: renal impairment, creatinine clearance renal decreased, blood creatinine increased, hematuria
Respiratory, thoracic and mediastinal disorders: cough, dyspnea
Skin and subcutaneous tissues disorders: dermatitis, night sweats, pruritus
Vascular disorders: hypotension
Other adverse reactions with valganciclovir hydrochloride in clinical trials in either patients with CMV retinitis or solid organ transplant patients that occurred in less than 5% of patients are listed below.
Blood and lymphatic disorders: febrile neutropenia, pancytopenia, bone marrow failure (including aplastic anemia)
Cardiovascular disorders: arrhythmia
Ear and labyrinth disorders: deafness
Eye disorders: macular edema
Gastrointestinal disorders: pancreatitis
Hemorrhage: potentially life-threatening bleeding associated with thrombocytopenia
Immune system disorders: hypersensitivity
Infections and infestations: cellulitis, sepsis
Injury, poisoning, and procedural complications: postoperative pain, wound dehiscence
Investigations: aspartate aminotransferase increased, alanine aminotransferase increased Musculoskeletal and connective tissue disorders: limb pain
Nervous system disorders: seizure, dysguesia (taste disturbance)
Psychiatric disorders: confusional state, agitation, psychotic disorder, hallucinations
Renal and urinary disorders: renal failure
Adverse Reactions in Pediatric Patients:
Valganciclovir for oral solution and tablets have been studied in 179 pediatric solid organ transplant patients who were at risk for developing CMV disease (aged 3 weeks to 16 years) and in 24 neonates with symptomatic congenital CMV disease (aged 8 to 34 days), with duration of ganciclovir exposure ranging from 2 to 200 days [see Use in Specific Populations (8.4), Clinical Studies (14.2)].
Prevention of CMV Disease in Pediatric Solid Organ Transplant Patients: The most frequently reported adverse reactions (greater than 10% of patients), regardless of seriousness, in pediatric solid organ transplant patients taking valganciclovir hydrochloride until Day 100 post-transplant were diarrhea, pyrexia, upper respiratory tract infection, vomiting, anemia, neutropenia, constipation and nausea. The most frequently reported adverse reactions (greater than 10% of patients) in pediatric kidney transplant patients treated with valganciclovir until Day 200 post-transplant were upper respiratory tract infection, urinary tract infection, diarrhea, leukopenia, neutropenia, headache, abdominal pain, tremor, pyrexia, anemia, blood creatinine increased, vomiting, and hematuria.
In general, the safety profile was similar in pediatric patients compared to that observed in adult patients. However, the rates of certain adverse reactions, and laboratory abnormalities, such as upper respiratory tract infection, pyrexia, nasopharyngitis, anemia, and abdominal pain were reported more frequently in pediatric patients than in adults [see Use in Specific Populations (8.4), Clinical Studies (14.2)]. Neutropenia was reported at a higher incidence in the two pediatric studies as compared to adults, but there was no correlation between neutropenia and infections observed in the pediatric population.
The overall safety profile of valganciclovir hydrochloride was similar with the extension of prophylaxis until Day 200 post-transplant in high risk pediatric kidney transplant patients. However, the incidence of severe neutropenia (ANC < 500/μL) was higher in pediatric kidney transplant patients treated with valganciclovir hydrochloride until Day 200 (17/57, 30%) compared to pediatric kidney transplant patients treated until Day 100 (3/63, 5%). There were no differences in the incidence of severe (Grade 4) anemia or thrombocytopenia in patients treated 100 or 200 days with valganciclovir hydrochloride.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of valganciclovir hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. As valganciclovir hydrochloride is rapidly and extensively converted to ganciclovir, any adverse reactions associated with ganciclovir might also occur with valganciclovir.
- Anaphylactic reaction
- Agranulocytosis
- Granulocytopenia
In general, the adverse reactions reported during the postmarketing use of valganciclovir hydrochloride were similar to those identified during the clinical trials.
7 DRUG INTERACTIONS
In vivo drug-drug interaction studies were not conducted with valganciclovir. However, because valganciclovir is rapidly and extensively converted to ganciclovir, drug-drug interactions associated with ganciclovir will be expected for valganciclovir hydrochloride. Drug-drug interaction studies with ganciclovir were conducted in patients with normal renal function. Following concomitant administration of valganciclovir hydrochloride and other renally excreted drugs, patients with impaired renal function may have increased concentrations of ganciclovir and the coadministered drug. Therefore, these patients should be closely monitored for toxicity of ganciclovir and the coadministered drug.
Established and other potentially significant drug interactions conducted with ganciclovir are listed in Table 9.
Table 9 Established and Other Potentially Significant Drug Interactions with Ganciclovir
| Name of the Concomitant
Drug | Change in the Concentration of Ganciclovir or Concomitant Drug
| Clinical Comment
|
| Imipenem-cilastatin | Unknown | Coadministration with imipenem-cilastatin is not recommended because generalized seizures have been reported in patients who received ganciclovir and imipenem-cilastatin. |
| Cyclosporine or amphotericin B | Unknown | Monitor renal function when valganciclovir hydrochloride is coadministered with cyclosporine or amphotericin B because of potential increase in serum creatinine [see Warnings and Precautions (5.2)].
|
| Mycophenolate mofetil (MMF) | ↔ Ganciclovir (in patients with normal renal function) ↔ MMF (in patients with normal renal function) | Based on increased risk, patients should be monitored for hematological and renal toxicity. |
| Other drugs associated with myelosuppression or nephrotoxicity (e.g., adriamycin, dapsone, doxorubicin, flucytosine, hydroxyurea, pentamidine, tacrolimus, trimethoprim/ sulfamethoxazole, vinblastine, vincristine, and zidovudine) | Unknown | Because of potential for higher toxicity, coadministration with valganciclovir hydrochloride should be considered only if the potential benefits are judged to outweigh the risks. |
| Didanosine | ↔ Ganciclovir ↑ Didanosine | Patients should be closely monitored for didanosine toxicity (e.g., pancreatitis) |
| Probenecid | ↑ Ganciclovir | Valganciclovir hydrochloride dose may need to be reduced. Monitor for evidence of ganciclovir toxicity. |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
After oral administration, valganciclovir (prodrug) is converted to ganciclovir (active drug) and, therefore, valganciclovir hydrochloride is expected to have reproductive toxicity effects similar to ganciclovir. In animal studies, ganciclovir caused maternal and fetal toxicity and embryo-fetal mortality in pregnant mice and rabbits as well as teratogenicity in rabbits at exposures two-times the human exposure. There are no available human data on use of valganciclovir hydrochloride or ganciclovir in pregnant women to establish the presence or absence of drug-associated risk. The background risk of major birth defects and miscarriage for the indicated populations is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4% and the risk of miscarriage is 15 to 20% of clinically recognized pregnancies. Advise pregnant women of the potential risk to the fetus [see Warnings and Precautions (5.3), Use in Specific Populations (8.3)].
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Most maternal CMV infections are asymptomatic or they may be associated with a self-limited mononucleosis-like syndrome. However, in immunocompromised patients (i.e., transplant patients or patients with AIDS) CMV infections may be symptomatic and may result in significant maternal morbidity and mortality. The transmission of CMV to the fetus is a result of maternal viremia and transplacental infection. Perinatal infection can also occur from exposure of the neonate to CMV shedding in the genital tract. Approximately 10% of children with congenital CMV infection are symptomatic at birth. Mortality in these infants is about 10% and approximately 50 to 90% of symptomatic surviving newborns experience significant morbidity, including mental retardation, sensorineural hearing loss, microcephaly, seizures, and other medical problems. The risk of congenital CMV infection resulting from primary maternal CMV infection may be higher and of greater severity than that resulting from maternal reactivation of CMV infection.
Data
Animal Data
Doses resulting in two-times the human exposure of ganciclovir (based on the human AUC following a single intravenous infusion of 5 mg per kg of ganciclovir) resulted in maternal and embryo-fetal toxicity in pregnant mice and rabbits as well as teratogenicity in the rabbits. Fetal resorptions were present in at least 85% of rabbits and mice. Rabbits showed increased embryo-fetal mortality, growth retardation of the fetuses and structural abnormalities of multiple organs of the fetuses including the palate (cleft palate), eyes (anophthalmia/microphthalmia), brain (hydrocephalus), jaw (brachygnathia), kidneys and pancreas (aplastic organs). Increased embryo-fetal mortality was also seen in mice. Daily intravenous doses of approximately 1.7 times the human exposure (based on AUC) administered to female mice prior to mating, during gestation, and during lactation caused hypoplasia of the testes and seminal vesicles in the male offspring, as well as pathologic changes in the nonglandular region of the stomach.
Data from an ex-vivo human placental model showed that ganciclovir crosses the human placenta. The transfer occurred by passive diffusion and was not saturable over a concentration range of 1 to 10 mg/mL.
8.2 Lactation
Risk Summary
No data are available regarding the presence of valganciclovir (prodrug) or ganciclovir (active drug) in human milk, the effects on the breastfed infant, or the effects on milk production. Animal data indicate that ganciclovir is excreted in the milk of lactating rats. The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Advise nursing mothers that breastfeeding is not recommended during treatment with valganciclovir hydrochloride because of the potential for serious adverse events in nursing infants and because of the potential for transmission of HIV [see Boxed Warning, Warnings and Precautions (5.1, 5.3, 5.4, 5.5), Nonclinical Toxicology (13.1)].
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Females of reproductive potential should undergo pregnancy testing before initiation of valganciclovir hydrochloride [see Use in Specific Populations (8.1)].
Contraception
Females
Because of the mutagenic and teratogenic potential of valganciclovir hydrochloride, females of reproductive potential should be advised to use effective contraception during treatment and for at least 30 days following treatment with valganciclovir hydrochloride [see Dosage and Administration (2.6), Warnings and Precautions (5.4, 5.5), Nonclinical Toxicology (13.1)].
Males
Because of its mutagenic potential, males should be advised to use condoms during and for at least 90 days following, treatment with valganciclovir hydrochloride [see Dosage and Administration (2.6), Warnings and Precautions (5.3, 5.5), Nonclinical Toxicology (13.1)].
Infertility
Valganciclovir hydrochloride at the recommended doses may cause temporary or permanent female and male infertility [see Warnings and Precautions (5.3), Nonclinical Toxicology (13.1)].
Data
Human Data
In a small, open-label, non-randomized clinical study, adult male renal transplant patients receiving valganciclovir hydrochloride for CMV prophylaxis for up to 200 days post-transplantation were compared to an untreated control group. Patients were followed-up for six months after valganciclovir hydrochloride discontinuation. Among 24 evaluable patients in the valganciclovir hydrochloride group, the mean sperm density at the end of treatment visit decreased by 11 million/mL from baseline; whereas, among 14 evaluable patients in the control group the mean sperm density increased by 33 million/mL. However, at the follow-up visit among 20 evaluable patients in the valganciclovir hydrochloride group the mean sperm density was comparable to that observed among 10 evaluable patients in the untreated control group (the mean sperm density at the end of follow-up visit increased by 41 million/mL from baseline in the valganciclovir hydrochloride group and by 43 million/mL in the untreated group).
8.4 Pediatric Use
Valganciclovir for oral solution and tablets are indicated for the prevention of CMV disease in pediatric kidney transplant patients 4 months to 16 years of age and in pediatric heart transplant patients 1 month to 16 years of age at risk for developing CMV disease [see Indications and Usage (1.2), Dosage and Administration (2.3)].
The use of valganciclovir for oral solution and tablets for the prevention of CMV disease in pediatric kidney transplant patients 4 months to 16 years of age is based on two single-arm, open-label, non-comparative studies in patients 4 months to 16 years of age. Study 1 was a safety and pharmacokinetic study in pediatric solid organ transplant patients (kidney, liver, heart, and kidney/pancreas). Valganciclovir hydrochloride was administered once daily within 10 days of transplantation for a maximum of 100 days post-transplantation. Study 2 was a safety and tolerability study where valganciclovir hydrochloride was administered once daily within 10 days of transplantation for a maximum of 200 days post-transplantation in pediatric kidney transplant patients. The results of these studies were supported by previous demonstration of efficacy in adult patients [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.2)].
The use of valganciclovir for oral solution and tablets for the prevention of CMV disease in pediatric heart transplant patients 1 month to 16 years of age is based on two studies (Study 1 described above and Study 3) and was supported by previous demonstration of efficacy in adult patients [see Clinical Pharmacology (12.3), Clinical Studies (14.2)]. Study 3 was a pharmacokinetic and safety study of valganciclovir hydrochloride in pediatric heart transplant patients less than 4 months of age who received a single dose of valganciclovir hydrochloride oral solution on each of two consecutive days. A physiologically based pharmacokinetic (PBPK) model was developed based on the available pharmacokinetic data from pediatric and adult patients to support dosing in heart transplant patients less than 1 month of age. However, due to uncertainty in model predictions for neonates, valganciclovir hydrochloride is not indicated for prophylaxis in this age group.
The safety and efficacy of valganciclovir for oral solution and tablets have not been established in children for prevention of CMV disease in pediatric liver transplant patients, in kidney transplant patients less than 4 months of age, in heart transplant patients less than 1 month of age, in pediatric AIDS patients with CMV retinitis, and in infants with congenital CMV infection.
A pharmacokinetic and pharmacodynamic evaluation of valganciclovir for oral solution was performed in 24 neonates with congenital CMV infection involving the central nervous system. All patients were treated for 6 weeks with a combination of intravenous ganciclovir 6 mg per kg twice daily or valganciclovir for oral solution at doses ranging from 14 mg per kg to 20 mg per kg twice daily. The pharmacokinetic results showed that in infants greater than 7 days to 3 months of age, a dose of 16 mg per kg twice daily of valganciclovir for oral solution provided ganciclovir systemic exposures (median AUC0 to 12h=23.6 [range 16.8 to 35.5] mcg∙h/mL; n=6) comparable to those obtained in infants up to 3 months of age from a 6 mg per kg dose of intravenous ganciclovir twice daily (AUC0 to 12h=25.3 [range 2.4 to 89.7] mcg∙h/mL; n=18) or to the ganciclovir systemic exposures obtained in adults from a 900 mg dose of valganciclovir tablets twice daily. However, the efficacy and safety of intravenous ganciclovir and of valganciclovir for oral solution have not been established for the treatment of congenital CMV infection in infants and no similar disease occurs in adults; therefore, efficacy cannot be extrapolated from intravenous ganciclovir use in adults.
8.5 Geriatric Use
Studies of valganciclovir for oral solution or tablets have not been conducted in adults older than 65 years of age. Clinical studies of valganciclovir hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Valganciclovir hydrochloride is known to be substantially excreted by the kidneys, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because renal clearance decreases with age, valganciclovir hydrochloride should be administered with consideration of their renal status. Renal function should be monitored and dosage adjustments should be made accordingly [see Dosage and Administration (2.5), Warnings and Precautions (5.2), Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Dose reduction is recommended when administering valganciclovir hydrochloride to patients with renal impairment [see Dosage and Administration (2.5), Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
For adult patients on hemodialysis (CrCl less than 10 mL/min), valganciclovir tablets should not be used. Adult hemodialysis patients should use ganciclovir in accordance with the dose-reduction algorithm cited in the CYTOVENE®-IV complete product information section on DOSAGE AND ADMINISTRATION: Renal Impairment [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Experience with Valganciclovir Tablets: An overdose of valganciclovir hydrochloride could possibly result in increased renal toxicity [see Dosage and Administration (2.5), Use in Specific Populations (8.6)]. Because ganciclovir is dialyzable, dialysis may be useful in reducing serum concentrations in patients who have received an overdose of valganciclovir hydrochloride [see Clinical Pharmacology (12.3)]. Adequate hydration should be maintained. The use of hematopoietic growth factors should be considered [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
Reports of adverse reactions after overdoses with valganciclovir, some with fatal outcomes, have been received from clinical trials and during postmarketing experience. The majority of patients experienced one or more of the following adverse events:
Hematological toxicity: myelosuppression including pancytopenia, bone marrow failure, leukopenia, neutropenia, granulocytopenia
Hepatotoxicity: hepatitis, liver function disorder
Renal toxicity: worsening of hematuria in a patient with pre-existing renal impairment, acute kidney injury, elevated creatinine
Gastrointestinal toxicity: abdominal pain, diarrhea, vomiting
Neurotoxicity: generalized tremor, seizure
11 DESCRIPTION
Valganciclovir for oral solution contains valganciclovir hydrochloride USP, a hydrochloride salt of the L-valyl ester of ganciclovir that exists as a mixture of two diastereomers. Ganciclovir is a synthetic guanine derivative active against CMV.
Valganciclovir hydrochloride is available as a powder for oral solution, which when constituted with water as directed contains 50 mg/mL valganciclovir free base. The inactive ingredients of valganciclovir for oral solution are mannitol, povidone, propylene glycol, saccharin sodium, sodium benzoate, tartaric acid, and tutti-frutti flavor.
Valganciclovir hydrochloride is a white to off-white powder with a molecular formula of C14H22N6O5·HCl and a molecular weight of 390.83. The chemical name for valganciclovir hydrochloride is L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester, monohydrochloride. Valganciclovir hydrochloride is a polar hydrophilic compound with a solubility of 70 mg/mL in water at 25ºC at a pH of 7.0 and an n-octanol/water partition coefficient of 0.0095 at pH 7.0. The pKa for valganciclovir hydrochloride is 7.6.
The chemical structure of valganciclovir hydrochloride is:

All doses in this insert are specified in terms of valganciclovir.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Valganciclovir is an antiviral drug with activity against CMV [see Microbiology (12.4)].
12.3 Pharmacokinetics
Valganciclovir is a prodrug of ganciclovir. Valganciclovir Cmax and AUC are approximately 1% and 3% of those of ganciclovir, respectively.
Pharmacokinetics in Adults: The pharmacokinetics of ganciclovir after administration of valganciclovir tablets have been evaluated in HIV- and CMV-seropositive patients, patients with AIDS and CMV retinitis, and in solid organ transplant patients (Table 10).
Table 10 Ganciclovir Pharmacokinetics* in Healthy Volunteers and HIV-positive/CMV-positive Adults Administered Valganciclovir Tablets 900 mg Once Daily with Food
| *Data were obtained from single and multiple dose studies in healthy volunteers, HIV-positive patients, and HIV-positive/CMV-positive patients with and without retinitis. Patients with CMV retinitis tended to have higher ganciclovir plasma concentrations than patients without CMV retinitis. | ||
| PK parameter
| N
| Value (Mean ± SD)
|
| AUC0 to 24h (mcg∙h/mL) | 57 | 29.1 ± 9.7 |
| Cmax (mcg/mL) | 58 | 5.61 ± 1.52 |
| Absolute oral bioavailability (%) | 32 | 59.4 ± 6.1 |
| Elimination half-life (hr) | 73 | 4.08 ± 0.76 |
| Renal clearance (mL/min/kg) | 20 | 3.21 ± 0.75 (1 study, n=20) |
The systemic ganciclovir exposures attained following administration of 900 mg valganciclovir tablets once daily were comparable across kidney, heart and liver transplant recipients (Table 11).
Table 11 Ganciclovir Pharmacokinetics in Solid Organ Transplant Recipients Administered Valganciclovir Tablets 900 mg Once Daily with Food
| *Includes kidney-pancreas | |||
| Parameter
| Value (Mean ± SD)
|
||
| Heart Transplant Recipients
(N=17) | Liver Transplant Recipients
(N=75) | Kidney Transplant Recipients*
(N=68) |
|
| AUC0 to 24h (mcg∙h/mL) | 40.2 ± 11.8 | 46.0 ± 16.1 | 48.2 ± 14.6 |
| Cmax (mcg/mL) | 4.9 ± 1.1 | 5.4 ± 1.5 | 5.3 ± 1.5 |
| Elimination half-life (hr) | 6.58 ± 1.50 | 6.18 ± 1.42 | 6.77 ± 1.25 |
The pharmacokinetic parameters of ganciclovir following 200 days of valganciclovir hydrochloride administration in high-risk kidney transplant patients were similar to those in solid organ transplant patients who received valganciclovir hydrochloride for 100 days.
Absorption, Distribution, Metabolism, and Excretion
The pharmacokinetic (PK) properties of valganciclovir hydrochloride are provided in Table 12.
Table 12 Pharmacokinetic Properties of Ganciclovir and Valganciclovir Associated with Valganciclovir Tablets
| aSteady state ganciclovir PK was assessed after administration of valganciclovir tablets (875 mg once daily) with a high fat meal containing approximately 600 total calories (31.1 g fat, 51.6 g carbohydrates and 22.2 g protein) to 16 HIV-positive subjects. | ||
| Valganciclovir
| Ganciclovir
|
|
| Absorption
|
||
| Tmax (h) median (min-max) (fed conditions) | | 2.18 1.7h to 3.0h |
| Food effect (high fat meal/fasting): PK parameter ratio and 90% confidence intervala
| | Cmax: 1.14 (0.95, 1.36) AUC: 1.30 (1.07, 1.51)a Tmax: ↔ |
| Distribution
|
||
| % Bound to human plasma proteins (ex vivo) | Unknown | 1 to 2% over 0.5 to 51 mcg/mL |
| Cerebrospinal fluid penetration | Unknown | Yes |
| Metabolism
|
||
| | Hydrolyzed by intestinal and liver esterases |
No significant metabolism |
| Elimination
|
||
| Dose proportionality | | AUC was dose proportional under fed conditions across a valganciclovir dose range of 450 to 2625 mg |
| Major route of elimination | Metabolism to ganciclovir | Glomerular filtration and active tubular secretion |
| t1/2 (h) | | See Tables 10 and 11 |
| % Of dose excreted in urine | Unknown |
|
| % Of dose excreted in feces | Unknown |
|
Specific Populations:
Renal Impairment: The pharmacokinetics of ganciclovir from a single oral dose of 900 mg valganciclovir tablets were evaluated in 24 otherwise healthy individuals with renal impairment. Decreased renal function results in decreased clearance of ganciclovir and increased terminal half-life (Table 13).
Table 13 Pharmacokinetics of Ganciclovir from a Single Oral Dose of 900 mg Valganciclovir Tablets
| *Creatinine clearance calculated from 24-hour urine collection. | ||||
| Estimated Creatinine Clearance*
(mL/min) | N
| Apparent Clearance (mL/min)
Mean ± SD | AUClast
(mcg∙h/mL) Mean ± SD | Half-life
(hours) Mean ± SD |
| 51 to 70 21 to 50 11 to 20 ≤ 10 | 6 6 6 6 | 249 ± 99 136 ± 64 45 ± 11 12.8 ± 8 | 49.5 ± 22.4 91.9 ± 43.9 223 ± 46 366 ± 66 | 4.85 ± 1.4 10.2 ± 4.4 21.8 ± 5.2 67.5 ± 34 |
Hemodialysis reduces plasma concentrations of ganciclovir by about 50% following valganciclovir hydrochloride administration. Adult patients receiving hemodialysis (CrCl less than 10 mL/min) cannot use valganciclovir tablets because the daily dose of valganciclovir tablets required for these patients is less than 450 mg [see Dosage and Administration (2.5) and Use in Specific Populations (8.6)].
Pharmacokinetics in Pediatric Patients: The pharmacokinetics of ganciclovir were evaluated following the administration of valganciclovir in 63 pediatric solid organ transplant patients aged 4 months to 16 years, and in 16 pediatric heart transplant patients less than 4 months of age. In these studies, patients received oral doses of valganciclovir (either valganciclovir for oral solution or tablets) to produce exposure equivalent to an adult 900 mg dose [see Dosage and Administration (2.3), Adverse Reactions (6.1), Use in Specific Populations (8.4), Clinical Studies (14.2)].
In studies using the pediatric valganciclovir dosing algorithm, the pharmacokinetics of ganciclovir were similar across organ types and age ranges (Table 14). Relative to adult transplant patients (Table 11), AUC values in pediatric patients were somewhat increased, but were within the range considered safe and effective in adults.
Table 14 Ganciclovir Pharmacokinetics by Age in Pediatric Solid Organ Transplant Patients Administered Valganciclovir Hydrochloride
| N=number of patients, NA=not applicable a Ages ranged from 26 to 124 days. |
|||||
| Organ
| PK Parameter mean (SD)
| < 4 months
| Age Group
4 months to ≤ 2 years | > 2 to < 12 years
| ≥ 12 years
|
| Heart
(N=26) | N AUC0 to 24h (mcg∙h/mL) Cmax (mcg/mL) t1/2 (h) | 14a
66.3 (20.5) 10.8 (3.30) 3.5 (0.87) | 6 55.4 (22.8) 8.2 (2.5) 3.8 (1.7) | 2 59.6 (21.0) 12.5 (1.2) 2.8 (0.9) | 4 60.6 (25.0) 9.5 (3.3) 4.9 (0.8) |
| Kidney
(N=31) | N AUC0 to 24h (mcg∙h/mL) Cmax (mcg/mL) t1/2 (h) | NA | 2 67.6 (13.0) 10.4 (0.4) 4.5 (1.5) | 10 55.9 (12.1) 8.7 (2.1) 4.8 (1.0) | 19 47.8 (12.4) 7.7 (2.1) 6.0 (1.3) |
| Liver
(N=17) | N AUC0 to 24h (mcg∙h/mL) Cmax (mcg/mL) t1/2 (h) | NA | 9 69.9 (37.0) 11.9 (3.7) 2.8 (1.5) | 6 59.4 (8.1) 9.5 (2.3) 3.8 (0.7) | 2 35.4 (2.8) 5.5 (1.1) 4.4 (0.2) |
Pharmacokinetics in Geriatric Patients: The pharmacokinetic characteristics of valganciclovir hydrochloride in elderly patients have not been established.
Drug Interactions: In vivo drug-drug interaction studies were not conducted with valganciclovir. However, because valganciclovir is rapidly and extensively converted to ganciclovir, interactions associated with ganciclovir will be expected for valganciclovir hydrochloride [see Drug Interactions (7)].
Table 15 and Table 16 provide a listing of established drug interaction studies with ganciclovir. Table 15 provides the effects of coadministered drug on ganciclovir plasma pharmacokinetic parameters, whereas Table 16 provides the effects of ganciclovir on plasma pharmacokinetic parameters of coadministered drug.
Table 15 Results of Drug Interaction Studies with Ganciclovir: Effects of Coadministered Drug on Ganciclovir Pharmacokinetic Parameters
| Coadministered Drug
| Ganciclovir Dosage
| N
| Ganciclovir Pharmacokinetic (PK) Parameter
|
| Mycophenolate mofetil (MMF) 1.5 g single dose | 5 mg/kg IV single dose | 12 | No effect on ganciclovir PK parameters observed (patients with normal renal function) |
| Trimethoprim 200 mg once daily | 1000 mg every 8 hours | 12 | No effect on ganciclovir PK parameters observed |
| Didanosine 200 mg every 12 hours simultaneously administered with ganciclovir | 5 mg/kg IV twice daily | 11 | No effect on ganciclovir PK parameters observed |
| 5 mg/kg IV once daily | 11 | No effect on ganciclovir PK parameters observed |
|
| Probenecid 500 mg every 6 hours | 1000 mg every 8 hours | 10 | AUC ↑ 53 ± 91% (range: -14% to 299%) Ganciclovir renal clearance ↓ 22 ± 20% (range: -54% to -4%) |
Table 16 Results of Drug Interaction Studies with Ganciclovir: Effects of Ganciclovir on Pharmacokinetic Parameters of Coadministered Drug
| Coadministered Drug
| Ganciclovir Dosage
| N
| Coadministered Drug Pharmacokinetic (PK) Parameter
|
| Oral cyclosporine at therapeutic doses | 5 mg/kg infused over 1 hour every 12 hours | 93 | In a retrospective analysis of liver allograft recipients, there was no evidence of an effect on cyclosporine whole blood concentrations. |
| Mycophenolate mofetil (MMF) 1.5 g single dose | 5 mg/kg IV single dose | 12 | No PK interaction observed (patients with normal renal function) |
| Trimethoprim 200 mg once daily | 1000 mg every 8 hours | 12 | No effect on trimethoprim PK parameters observed |
| Didanosine 200 mg every 12 hours | 5 mg/kg IV twice daily | 11 | AUC0 to 12 ↑70 ± 40% (range: 3% to 121%) Cmax↑49 ± 48% (range: -28% to 125%) |
| Didanosine 200 mg every 12 hours | 5 mg/kg IV once daily | 11 | AUC0 to 12 ↑50 ± 26% (range: 22% to 110%) Cmax ↑36 ± 36% (range: -27% to 94%) |
12.4 Microbiology
Mechanism of Action: Valganciclovir is an L-valyl ester (prodrug) of ganciclovir that exists as a mixture of two diastereomers. After oral administration, both diastereomers are rapidly converted to ganciclovir by intestinal and hepatic esterases. Ganciclovir is a synthetic analogue of 2'-deoxyguanosine, which inhibits replication of human CMV in cell culture and in vivo.
In CMV-infected cells, ganciclovir is initially phosphorylated to ganciclovir monophosphate by the viral protein kinase, pUL97. Further phosphorylation occurs by cellular kinases to produce ganciclovir triphosphate, which is then slowly metabolized intracellularly (half-life 18 hours). As the phosphorylation is largely dependent on the viral kinase, phosphorylation of ganciclovir occurs preferentially in virus-infected cells. The virustatic activity of ganciclovir is due to inhibition of the viral DNA polymerase, pUL54 by ganciclovir triphosphate.
Antiviral Activity: The quantitative relationship between the cell culture susceptibility of human herpes viruses to antivirals and clinical response to antiviral therapy has not been established, and virus sensitivity testing has not been standardized. Sensitivity test results, expressed as the concentration of drug required to inhibit the growth of virus in cell culture by 50% (EC50), vary greatly depending upon a number of factors including the assay used. Thus, the reported EC50 values of ganciclovir that inhibit human CMV replication in cell culture (laboratory and clinical isolates) have ranged from 0.08 to 22.94 μM (0.02 to 5.75 mcg/mL). The distribution and range in susceptibility observed in one assay evaluating 130 clinical isolates was 0 to 1 μM (35%), 1.1 to 2 μM (20%), 2.1 to 3 μM (27%), 3.1 to 4 μM (13%), 4.1 to 5 μM (5%), less than 5 μM (less than 1%). Ganciclovir inhibits mammalian cell proliferation (CC50) in cell culture at higher concentrations ranging from 40 to greater than 1,000 μM (10.21 to greater than 250 mcg/mL). Bone marrow-derived colony-forming cells are more sensitive [CC50 value = 2.7 to 12 μM (0.69 to 3.06 mcg/mL)].
Viral Resistance:
Cell culture: CMV isolates with reduced susceptibility to ganciclovir have been selected in cell culture. Growth of CMV strains in the presence of ganciclovir resulted in the selection of amino acid substitutions in the viral protein kinase pUL97 (M460I/V, L595S, G598D, and K599T) and the viral DNA polymerase pUL54 (D301N, N410K, F412V, P488R, L516R, C539R, L545S, F595I, V812L, P829S, L862F, D879G, and V946L).
In vivo: Viruses resistant to ganciclovir can arise after prolonged treatment or prophylaxis with valganciclovir by selection of substitutions in pUL97 and/or pUL54. Limited clinical data are available on the development of clinical resistance to ganciclovir and many pathways to resistance likely exist. In clinical isolates, seven canonical pUL97 substitutions, (M460V/I, H520Q, C592G, A594V, L595S, and C603W) are the most frequently reported ganciclovir resistance-associated substitutions. These and other substitutions less frequently reported in the literature, or observed in clinical trials, are listed in Table 17.
Table 17 Summary of Resistance-associated Amino Acid Substitutions Observed in the CMV of Patients Failing Ganciclovir Treatment or Prophylaxis
| pUL97 | F342Y, K359E/Q, L405P, A440V, M460I/V/T/L, V466G/M, C480F, C518Y, H520Q, P521L, del 590-593, A591D/V, C592F/G, A594E/G/T/V/P, L595F/S/T/W, del 595, del 595-603, E596D/G/Y, K599E/M, del 600-601, del 597-600, del 601-603, C603W/R/S/Y, C607F/S/Y, I610T, A613V |
| pUL54 | E315D, N408D/K/S, F412C/L/S, D413A/E/N, L501F/I, T503I, K513E/N/R, D515E, L516W, I521T, P522A/L/S, V526L, C539G, L545S/W, Q578H/L, D588E/N, G629S, S695T, I726T/V, E756K, L773V, V781I, V787E/L, L802M, A809V, T813S, T821I, A834P, G841A/S, D879G, A972V, del 981-982, A987G |
Note: Many additional pathways to ganciclovir resistance likely exist
The presence of known ganciclovir resistance-associated amino acid substitutions was evaluated in a study that extended valganciclovir CMV prophylaxis from 100 days to 200 days post-transplant in adult kidney transplant patients at high risk for CMV disease (D+/R-) [see Clinical Studies (14.1)]. Five subjects from the 100 day group and four subjects from the 200 day group meeting the resistance analysis criteria had known ganciclovir resistance-associated amino acid substitutions detected. In six subjects, the following resistance-associated amino acid substitutions were detected within pUL97: 100 day group: A440V, M460V, C592G; 200 day group: M460V, C603W. In three subjects, the following resistance-associated amino acid substitutions were detected within pUL54: 100 day group: E315D; 200 day group: E315D, P522S. Overall, the detection of known ganciclovir resistance-associated amino acid substitutions was observed more frequently in patients during prophylaxis therapy than after the completion of prophylaxis therapy (during therapy: 5/12 [42%] versus after therapy: 4/58 [7%]). The possibility of viral resistance should be considered in patients who show poor clinical response or experience persistent viral excretion during therapy.
Cross-Resistance: Cross-resistance has been reported for amino acid substitutions selected in cell culture by ganciclovir, cidofovir or foscarnet. In general, amino acid substitutions in pUL54 conferring cross-resistance to ganciclovir and cidofovir are located within the exonuclease domains and region V of the viral DNA polymerase. Whereas, amino acid substitutions conferring cross-resistance to foscarnet are diverse, but concentrate at and between regions II (codon 696 to 742) and III (codon 805 to 845). The amino acid substitutions that resulted in reduced susceptibility to ganciclovir and either cidofovir and/or foscarnet are summarized in Table 18.
Substitutions at amino acid positions pUL97 340 to 400 have been found to confer resistance to ganciclovir. Resistance data based on assays that do not include this region should be interpreted cautiously.
Table 18 Summary of pUL54 Amino Acid Substitutions with Cross-Resistance between Ganciclovir, Cidofovir, and/or Foscarnet
| Cross-resistant to cidofovir
| D301N, N408D/K, N410K, F412C/L/S/V, D413E/N, P488R, L501I, T503I, K513E/N, L516R/W, I521T, P522S/A, V526L, C539G/R, L545S/W, Q578H, D588N, I726T/V, E756K, L733V, V787E, V812L, T813S, A834P, G841A, del 981-982, A987G |
| Cross-resistant to foscarnet
| F412C, Q578H/L, D588N, V715A/M, E756K, L733V, V776M, V781I, V787E/L, L802M, A809V, V812L, T813S, T821I, A834P, G841A/S, del 981-982 |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies have not been conducted with valganciclovir hydrochloride. However, upon oral administration, valganciclovir is rapidly and extensively converted to ganciclovir. Therefore, like ganciclovir, valganciclovir is a potential carcinogen.
Ganciclovir was carcinogenic in the mouse at oral doses that produced exposures approximately 0.1x and 1.4x, respectively, the mean drug exposure in humans following the recommended intravenous dose of 5 mg/kg, based on area under the plasma concentration curve (AUC) comparisons. At the higher dose, there was a significant increase in the incidence of tumors of the preputial gland in males, forestomach (nonglandular mucosa) in males and females, and reproductive tissues (ovaries, uterus, mammary gland, clitoral gland and vagina) and liver in females. At the lower dose, a slightly increased incidence of tumors was noted in the preputial and harderian glands in males, forestomach in males and females, and liver in females. Ganciclovir should be considered a potential carcinogen in humans.
Valganciclovir increases mutations in mouse lymphoma cells. In the mouse micronucleus assay, valganciclovir was clastogenic. Valganciclovir was not mutagenic in the Ames Salmonella assay. Ganciclovir increased mutations in mouse lymphoma cells and DNA damage in human lymphocytes in vitro. In the mouse micronucleus assay, ganciclovir was clastogenic. Ganciclovir was not mutagenic in the Ames Salmonella assay.
Valganciclovir is converted to ganciclovir and therefore is expected to have similar reproductive toxicity effects as ganciclovir [see Warnings and Precautions (5.3)]. Ganciclovir caused decreased mating behavior, decreased fertility, and an increased incidence of embryolethality in female mice following intravenous doses that produced an exposure approximately 1.7x the mean drug exposure in humans following the dose of 5 mg per kg, based on AUC comparisons. Ganciclovir caused decreased fertility in male mice and hypospermatogenesis in mice and dogs following daily oral or intravenous administration. Systemic drug exposure (AUC) at the lowest dose showing toxicity in each species ranged from 0.03 to 0.1x the AUC of the recommended human intravenous dose. Valganciclovir caused similar effects on spermatogenesis in mice, rats, and dogs. These effects were reversible at lower doses but irreversible at higher doses. It is considered likely that ganciclovir (and valganciclovir) could cause temporary or permanent inhibition of human spermatogenesis.
14 CLINICAL STUDIES
14.1 Adult Patients
Induction Therapy of CMV Retinitis: In one randomized open-label controlled study, 160 patients with AIDS and newly diagnosed CMV retinitis were randomized to receive treatment with either valganciclovir tablets (900 mg twice daily for 21 days, then 900 mg once daily for 7 days) or with intravenous ganciclovir solution (5 mg per kg twice daily for 21 days, then 5 mg per kg once daily for 7 days). Study participants were: male (91%), White (53%), Hispanic (31%), and Black (11%). The median age was 39 years, the median baseline HIV-1 RNA was 4.9 log10, and the median CD4 cell count was 23 cells/mm3. A determination of CMV retinitis progression by the masked review of retinal photographs taken at baseline and Week 4 was the primary outcome measurement of the 3-week induction therapy. Table 19 provides the outcomes at 4 weeks.
Table 19 Week 4 Masked Review of Retinal Photographs in CMV Retinitis Study
| | Intravenous Ganciclovir
| Valganciclovir Tablets
|
| Determination of CMV retinitis progression at Week 4 | N=80 | N=80 |
| Progressor Non-progressor | 7 63 | 7 64 |
| Death Discontinuations due to Adverse Events Failed to return | 2 1 1 | 1 2 1 |
| CMV not confirmed at baseline or no interpretable baseline photos | 6 | 5 |
Maintenance Therapy of CMV Retinitis: No comparative clinical data are available on the efficacy of valganciclovir tablets for the maintenance therapy of CMV retinitis because all patients in the CMV retinitis study received open-label valganciclovir tablets after Week 4. However, the AUC for ganciclovir is similar following administration of 900 mg valganciclovir tablets once daily and 5 mg per kg intravenous ganciclovir once daily. Although the ganciclovir Cmax is lower following valganciclovir tablets administration compared to intravenous ganciclovir, it is higher than the Cmax obtained following oral ganciclovir administration. Therefore, use of valganciclovir tablets as maintenance therapy is supported by a plasma concentration-time profile similar to that of two approved products for maintenance therapy of CMV retinitis.
Prevention of CMV Disease in Heart, Kidney, Kidney-Pancreas, or Liver Transplantation: A double blind, double-dummy active comparator study was conducted in 372 heart, liver, kidney, or kidney-pancreas transplant patients at high risk for CMV disease (D+/R-). Patients were randomized (2 valganciclovir hydrochloride: 1 oral ganciclovir) to receive either valganciclovir tablets (900 mg once daily) or oral ganciclovir (1000 mg three times a day) starting within 10 days of transplantation until Day 100 post-transplant. The proportion of patients who developed CMV disease, including CMV syndrome and/or tissue-invasive disease during the first 6 months post-transplant was similar between the valganciclovir tablets arm (12.1%, N=239) and the oral ganciclovir arm (15.2%, N=125). However, in liver transplant patients, the incidence of tissue-invasive CMV disease was significantly higher in the valganciclovir hydrochloride group compared with the ganciclovir group. These results are summarized in Table 20.
Mortality at six months was 3.7% (9/244) in the valganciclovir hydrochloride group and 1.6% (2/126) in the oral ganciclovir group.
Table 20 Percentage of Patients with CMV Disease, Tissue-Invasive CMV Disease or CMV Syndrome by Organ Type: Endpoint Committee, 6 Month ITT Population
| | CMV Disease1
| Tissue-Invasive CMV Disease
| CMV Syndrome2
|
|||
| Organ
| VGCV | GCV | VGCV | GCV | VGCV | GCV |
| | (N=239) | (N=125) | (N=239) | (N=125) | (N=239) | (N=125) |
| Liver | 19% | 12% | 14% | 3% | 5% | 8% |
| (n=177) | (22/118) | (7/59) | (16/118) | (2/59) | (6/118) | (5/59) |
| Kidney | 6% | 23% | 1% | 5% | 5% | 18% |
| (n=120) | (5/81) | (9/39) | (1/81) | (2/39) | (4/81) | (7/39) |
| Heart | 6% | 10% | 0% | 5% | 6% | 5% |
| (n=56) | (2/35) | (2/21) | (0/35) | (1/21) | (2/35) | (1/21) |
| Kidney/Pancreas | 0% | 17% | 0% | 17% | 0% | 0% |
| (n=11) | (0/5) | (1/6) | (0/5) | (1/6) | (0/5) | (0/6) |
GCV = oral ganciclovir; VGCV = valganciclovir
1Number of patients with CMV disease = Number of patients with tissue-invasive CMV disease or CMV syndrome
2CMV syndrome was defined as evidence of CMV viremia accompanied with fever greater than or equal to 38°C on two or more occasions separated by at least 24 hours within a 7-day period and one or more of the following: malaise, leukopenia, atypical lymphocytosis, thrombocytopenia, and elevation of hepatic transaminases
Prevention of CMV Disease in Kidney Transplantation: A double-blind, placebo-controlled study was conducted in 326 kidney transplant patients at high risk for CMV disease (D+/R-) to assess the efficacy and safety of extending valganciclovir hydrochloride CMV prophylaxis from 100 to 200 days post-transplant. Patients were randomized (1:1) to receive valganciclovir tablets (900 mg once daily) within 10 days of transplantation either until Day 200 post-transplant or until Day 100 post-transplant followed by 100 days of placebo. Extending CMV prophylaxis with valganciclovir hydrochloride until Day 200 post-transplant demonstrated superiority in preventing CMV disease within the first 12 months post-transplant in high risk kidney transplant patients compared to the 100 day dosing regimen (primary endpoint). These results are summarized in Table 21.
Table 21 Percentage of Kidney Transplant Patients with CMV Disease, Tissue- Invasive CMV Disease or CMV Syndrome, 12 Month ITT Population
| | CMV Disease1 | Tissue-Invasive CMV Disease | CMV Syndrome2 |
|||
| | 100 Days VGCV (N=163) | 200 Days VGCV (N=155) | 100 Days VGCV (N=163) | 200 Days VGCV (N=155) | 100 Days VGCV (N=163) | 200 Days VGCV (N=155) |
| Cases | 36.8% (60/163) | 16.8% (26/155) | 1.8% (3/163)3
| 0.6% (1/155) | 35. 0% (57/163) | 16.1% (25/155) |
VGCV = valganciclovir.
1Number of patients with CMV disease = Number of patients with tissue-invasive CMV disease or CMV syndrome
2CMV syndrome was defined as evidence of CMV viremia accompanied with at least one of the following: fever (greater than or equal to 38ºC), severe malaise, leukopenia, atypical lymphocytosis, thrombocytopenia, and elevation of hepatic transaminases
3Two patients in the 100 day group had both tissue-invasive CMV disease and CMV syndrome; however, these patients are counted as having only tissue-invasive CMV disease.
The percentage of kidney transplant patients with CMV disease at 24 months post-transplant was 38.7% (63/163) for the 100 day dosing regimen and 21.3% (33/155) for the 200 day dosing regimen.
14.2 Pediatric Patients
Prevention of CMV in Pediatric Heart, Kidney, or Liver Transplantation: Sixty-three children, 4 months to 16 years of age, who had a solid organ transplant (kidney 33, liver 17, heart 12, and kidney/liver 1) and were at risk for developing CMV disease, were enrolled in an open-label, safety, and pharmacokinetic study of oral valganciclovir hydrochloride (valganciclovir for oral solution or tablets). Patients received valganciclovir hydrochloride once daily within 10 days after transplant until a maximum of 100 days post-transplant. The daily doses of valganciclovir hydrochloride were calculated at each study visit based on body surface area and a modified creatinine clearance [see Dosage and Administration (2.3)].
The pharmacokinetics of ganciclovir were similar across organ transplant types and age ranges. The mean daily ganciclovir exposures in pediatric patients were somewhat increased relative to those observed in adult solid organ transplant patients receiving valganciclovir hydrochloride 900 mg once daily, but were within the range considered safe and effective in adults [see Clinical Pharmacology (12.3)]. No case of CMV syndrome or tissue-invasive CMV disease was reported within the first six months post-transplantation.
Prevention of CMV in Pediatric Kidney Transplantation: Fifty-seven children, 1 to 16 years of age, who had a renal transplant and were at risk for developing CMV disease, were enrolled in an open-label tolerability study of oral valganciclovir hydrochloride (valganciclovir for oral solution or tablets). Patients received valganciclovir hydrochloride once daily within 10 days after transplant until a maximum of 200 days post-transplant. The daily doses of valganciclovir hydrochloride were calculated at each study visit based on body surface area and a modified creatinine clearance [see Dosage and Administration (2.3)]. No case of CMV syndrome or tissue-invasive CMV disease was reported within the first 12 months post-transplantation.
15 REFERENCES
- Brion LP, Fleischman AR, McCarton C, Schwartz GJ. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J of Ped 1986: 109(4): 698-707.
- NIOSH [2014]. NIOSH list of antineoplastic and other hazardous drugs in healthcare settings. By Connor TH, MacKenzie BA, DeBord DG, Trout DB, O’Callaghan JP, Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2014-138 (Supersedes 2012-150).
16 HOW SUPPLIED/STORAGE AND HANDLING
Valganciclovir for oral solution: Supplied as a white to off-white powder blend for constitution, forming a colorless to brownish yellow tutti-frutti flavored solution. Available in glass bottles containing approximately 100 mL of solution after constitution. Each bottle can deliver up to a total of 88 mL of solution. Each bottle is supplied with a bottle adapter and 2 oral dispensers (NDC 59651-444-01).
Prior to dispensing to the patient, valganciclovir for oral solution must be prepared by the pharmacist [see Dosage and Administration (2.4)].
Store dry powder at 20º to 25ºC (68º to 77ºF) [see USP Controlled Room Temperature].
Store constituted solution under refrigeration at 2º to 8ºC (36º to 46ºF) for no longer than 49 days. Do not freeze.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Adverse Reactions
Inform patients that valganciclovir hydrochloride may cause granulocytopenia (neutropenia), anemia, thrombocytopenia and elevated creatinine levels and that dose modification or discontinuation of dosing may be required. Complete blood counts, platelet counts, and creatinine levels should be monitored frequently during treatment [see Warnings and Precautions (5.1)].
Pregnancy and Contraception
Inform females of reproductive potential that valganciclovir hydrochloride causes birth defects in animals. Advise them to use effective contraception during and for at least 30 days following treatment with valganciclovir hydrochloride. Similarly, advise males to use condoms during and for at least 90 days following treatment with valganciclovir hydrochloride [see Use in Specific Populations (8.1, 8.3)].
Carcinogenicity
Advise patients that valganciclovir hydrochloride is considered a potential carcinogen [see Nonclinical Toxicity (13.1)].
Lactation
Advise mothers not to breast-feed if they are receiving valganciclovir hydrochloride because of the potential for hematologic toxicity and cancer in nursing infants, and because HIV can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].
Infertility
Advise patients that valganciclovir hydrochloride may cause temporary or permanent female and male infertility [see Warnings and Precautions (5.3), Use in Specific Populations (8.3)].
Impairment of Cognitive Ability
Inform patients that tasks requiring alertness may be affected including the patient’s ability to drive and operate machinery as seizures, dizziness, and/or confusion have been reported with the use of valganciclovir hydrochloride [see Adverse Reactions (6.1)].
Use in Patients with CMV Retinitis
Inform patients that valganciclovir hydrochloride is not a cure for CMV retinitis, and they may continue to experience progression of retinitis during or following treatment. Advise patients to have ophthalmologic follow-up examinations at a minimum of every 4 to 6 weeks while being treated with valganciclovir hydrochloride. Some patients will require more frequent follow-up.
Administration
Inform adult patients that they should use valganciclovir tablets, not valganciclovir for oral solution [see Dosage and Administration (2.1)].
Inform patients to take valganciclovir for oral solution with food to maximize bioavailability.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Issued: February 2022
For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876
Dispense with Patient Information available at: www.aurobindousa.com/medication-guides
SPL UNCLASSIFIED SECTION
|
PATIENT INFORMATION Valganciclovir for Oral Solution (val'' gan sye' kloe vir) |
| What is the most important information I should know about valganciclovir for oral solution?
Valganciclovir for oral solution can cause serious side effects, including:
|
| What is valganciclovir for oral solution?
Valganciclovir for oral solution is a prescription antiviral medicine. In adults, valganciclovir tablets are used:
In children, valganciclovir tablets or oral solution are used:
|
| Do not take valganciclovir for oral solution if you have had a serious allergic reaction to valganciclovir, ganciclovir or any of the ingredients of valganciclovir for oral solution. See the end of this leaflet for a list of the ingredients in valganciclovir for oral solution. |
Before you take valganciclovir for oral solution, tell your healthcare provider about all of your medical conditions, including if you:
|
How should I take valganciclovir for oral solution?
|
| What should I avoid during treatment with valganciclovir for oral solution?
Valganciclovir for oral solution can cause seizures, dizziness, and confusion. You should not drive a car or operate machinery until you know how valganciclovir for oral solution affects you. |
| What are the possible side effects of valganciclovir for oral solution?
Valganciclovir for oral solution may cause serious side effects, including: See “What is the most important information I should know about valganciclovir for oral solution?” The most common side effects of valganciclovir hydrochloride in adults include: • diarrhea • low white cell, red cell and platelet cell counts in blood tests • fever • headache • fatigue • sleeplessness • nausea • urinary tract infection • shaky movements (tremors) • vomiting The most common side effects of valganciclovir for oral solution in children include: • diarrhea • vomiting • fever • low white blood cell counts in blood tests • upper respiratory tract infection • headache • urinary tract infection These are not all the possible side effects of valganciclovir for oral solution. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
How should I store valganciclovir for oral solution?
|
| General information about the safe and effective use of valganciclovir for oral solution.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use valganciclovir for oral solution for a condition for which it was not prescribed. Do not give valganciclovir for oral solution to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about valganciclovir for oral solution that is written for health professionals. |
| What are the ingredients in valganciclovir for oral solution?
Active ingredient: valganciclovir hydrochloride Inactive ingredients for oral solution: mannitol, povidone, propylene glycol, saccharin sodium, sodium benzoate, tartaric acid, and tutti-frutti flavor. |
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Issued: February 2022
For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876.
Dispense with Patient Information available at: www.aurobindousa.com/medication-guides
SPL UNCLASSIFIED SECTION
Instructions for Use
Valganciclovir for Oral Solution
(val'' gan sye' kloe vir)
Be sure that you read, and that you understand and follow these instructions carefully to ensure proper dosing of the oral solution.
Important:
- Avoid contact with your skin or eyes. If you come in contact with the contents of the oral solution, wash your skin well with soap and water or rinse your eyes well with plain water.
- Do not use valganciclovir for oral solution after the discard date on the bottle.
- Always use the oral dispenser provided to give or take a dose of valganciclovir for oral solution.
- Call your pharmacist if your oral dispenser is lost or damaged, and they will tell you how to continue to give or take a dose of valganciclovir for oral solution.
- Ask your healthcare provider or pharmacist to show you how to measure your prescribed dose.
To take a dose of valganciclovir for oral solution, you will need the bottle of medicine and an oral dispenser provided with the medicine (see Figure 1). Your pharmacist inserts the bottle adapter in the valganciclovir for oral solution bottle.
Figure 1

| Step 1: With the child-resistant cap on the bottle, shake the bottle well for about 5 seconds before each use. |  |
| Step 2: Open the bottle by pressing downward firmly on the child-resistant cap and turning it counter-clockwise. Do not throw away the child-resistant cap.
|  |
| Step 3: Check the dose in milliliters (mL) as prescribed by your healthcare provider. Find this number on the oral dispenser. |  |
| Step 4: Push the plunger down toward the tip of the oral dispenser. |  |
| Step 5: With the bottle in an upright position, insert the oral dispenser into the bottle adapter opening until firmly in place. | 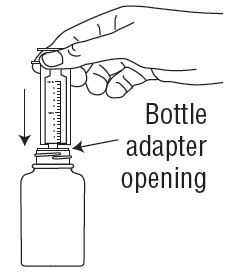 |
| Step 6: Carefully turn the bottle upside down with the oral dispenser in place. Pull the plunger to withdraw the prescribed dose. If you see air bubbles in the oral dispenser, fully push in the plunger so that the oral solution flows back into the bottle. Then withdraw your prescribed dose of valganciclovir for oral solution. |  |
| Step 7: Leave the oral dispenser in the bottle adapter and turn the bottle to an upright position. Slowly remove the oral dispenser from the bottle adapter. |  |
Step 8: Give or take the dose of valganciclovir for oral solution.
|  |
| Step 9: Put the child-resistant cap back on the bottle. Return the bottle back to the refrigerator. |  |
Step 10: Rinse the oral dispenser with tap water after each use.
|   |
How should I store valganciclovir for oral solution?
- Store solution in the refrigerator at 36°F to 46°F (2°C to 8°C) for no longer than 49 days.
- Do not freeze.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Issued: February 2022
For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL CONTAINER LABEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL CARTON LABEL
INGREDIENTS AND APPEARANCE
| VALGANCICLOVIR
valganciclovir hydrochloride powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Aurobindo Pharma Limited (650082092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 918917642 | ANALYSIS(59651-444) , MANUFACTURE(59651-444) | |