Search by Drug Name or NDC
NDC 61958-0901-01 Cayston Details
Cayston
Cayston is a KIT in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Gilead Sciences, Inc.. The primary component is .
Product Information
| NDC | 61958-0901 |
|---|---|
| Product ID | 61958-0901_e3d9f13f-c1a8-4024-b668-b1545293a6f7 |
| Associated GPIs | 16140010402120 |
| GCN Sequence Number | 065913 |
| GCN Sequence Number Description | aztreonam lysine VIAL-NEB 75 MG/ML INHALATION |
| HIC3 | W1P |
| HIC3 Description | BETALACTAMS |
| GCN | 28039 |
| HICL Sequence Number | 036792 |
| HICL Sequence Number Description | AZTREONAM LYSINE |
| Brand/Generic | Brand |
| Proprietary Name | Cayston |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | aztreonam |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | KIT |
| Route | n/a |
| Active Ingredient Strength | n/a |
| Active Ingredient Units | n/a |
| Substance Name | n/a |
| Labeler Name | Gilead Sciences, Inc. |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA050814 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 61958-0901-01 (61958090101)
| NDC Package Code | 61958-0901-1 |
|---|---|
| Billing NDC | 61958090101 |
| Package | 2 CARTON in 1 PACKAGE (61958-0901-1) / 1 KIT in 1 CARTON * 1 mL in 1 VIAL * 1 mL in 1 AMPULE |
| Marketing Start Date | 2010-02-22 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 67300ca3-8c53-4ce4-8e86-2c03be1f9b8a Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
CAYSTON® (aztreonam for inhalation solution), for oral inhalation use
Initial U.S. Approval: 1986
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CAYSTON and other antibacterial drugs, CAYSTON should be used only to treat patients with cystic fibrosis (CF) known to have Pseudomonas aeruginosa in the lungs. (1)
INDICATIONS AND USAGE
CAYSTON is a monobactam antibacterial indicated to improve respiratory symptoms in cystic fibrosis (CF) patients with Pseudomonas aeruginosa. Safety and effectiveness have not been established in pediatric patients below the age of 7 years, patients with FEV1 <25% or >75% predicted, or patients colonized with Burkholderia cepacia. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Contraindicated in patients with a known allergy to aztreonam. (4)
WARNINGS AND PRECAUTIONS
- Allergic reaction to CAYSTON was seen in clinical trials. Stop treatment if an allergic reaction occurs. Use caution when CAYSTON is administered to patients with a known allergic reaction to beta-lactams. (5.1)
- Bronchospasm has been reported with CAYSTON. Stop treatment if chest tightness develops during nebulizer use. (5.2)
ADVERSE REACTIONS
Common adverse reactions (more than 5%) occurring more frequently in CAYSTON patients are cough, nasal congestion, wheezing, pharyngolaryngeal pain, pyrexia, chest discomfort, abdominal pain and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD5, option 3 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Instructions for CAYSTON Reconstitution
2.3 Instructions for CAYSTON Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Allergic Reactions
5.2 Bronchospasm
5.3 Decreases in FEV1 After 28-Day Treatment Cycle
5.4 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
CAYSTON® is indicated to improve respiratory symptoms in cystic fibrosis (CF) patients with Pseudomonas aeruginosa. Safety and effectiveness have not been established in pediatric patients below the age of 7 years, patients with FEV1 <25% or >75% predicted, or patients colonized with Burkholderia cepacia [see Clinical Studies (14)].
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CAYSTON and other antibacterial drugs, CAYSTON should be used only to treat patients with CF known to have Pseudomonas aeruginosa in the lungs.
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
The recommended dose of CAYSTON for both adults and pediatric patients 7 years of age and older is one single-use vial (75 mg of aztreonam) reconstituted with 1 mL of sterile diluent administered 3 times a day for a 28-day course (followed by 28 days off CAYSTON therapy). Dosage is not based on weight or adjusted for age. Doses should be taken at least 4 hours apart.
CAYSTON is administered by inhalation using an Altera® Nebulizer System. Patients should use a bronchodilator before administration of CAYSTON.
2.2 Instructions for CAYSTON Reconstitution
CAYSTON should be administered immediately after reconstitution. Do not reconstitute CAYSTON until ready to administer a dose.
Take one amber glass vial containing CAYSTON and one diluent ampule from the carton. To open the glass vial, carefully remove the blue cap and metal ring and remove the gray rubber stopper. Twist the tip off the diluent ampule and squeeze the liquid into the glass vial. Replace the rubber stopper, then gently swirl the vial until contents have completely dissolved.
The empty vial, stopper, and diluent ampule should be disposed of properly upon completion of dosing.
2.3 Instructions for CAYSTON Administration
CAYSTON is administered by inhalation using an Altera Nebulizer System. CAYSTON should not be administered with any other nebulizer. CAYSTON should not be mixed with any other drugs in the Altera Nebulizer Handset.
CAYSTON is not for intravenous or intramuscular administration.
Patients should use a bronchodilator before administration of CAYSTON. Short-acting bronchodilators can be taken between 15 minutes and 4 hours prior to each dose of CAYSTON. Alternatively, long-acting bronchodilators can be taken between 30 minutes and 12 hours prior to administration of CAYSTON. For patients taking multiple inhaled therapies, the recommended order of administration is as follows: bronchodilator, mucolytics, and lastly, CAYSTON.
To administer CAYSTON, pour the reconstituted solution into the handset of the nebulizer system. Turn the unit on. Place the mouthpiece of the handset in your mouth and breathe normally only through your mouth. Administration typically takes between 2 and 3 minutes. Further patient instructions on how to administer CAYSTON are provided in the FDA-approved patient labeling. Instructions on testing nebulizer functionality and cleaning the handset are provided in the Instructions for Use included with the nebulizer system.
3 DOSAGE FORMS AND STRENGTHS
5 WARNINGS AND PRECAUTIONS
5.1 Allergic Reactions
Severe allergic reactions have been reported following administration of aztreonam for injection to patients with no known history of exposure to aztreonam. In addition, allergic reaction with facial rash, facial swelling, and throat tightness was reported with CAYSTON in clinical trials. If an allergic reaction to CAYSTON occurs, stop administration of CAYSTON and initiate treatment as appropriate.
Caution is advised when administering CAYSTON to patients if they have a history of beta-lactam allergy, although patients with a known beta-lactam allergy have received CAYSTON in clinical trials and no severe allergic reactions were reported. A history of allergy to beta-lactam antibiotics, such as penicillins, cephalosporins, and/or carbapenems, may be a risk factor, since cross-reactivity may occur.
5.2 Bronchospasm
Bronchospasm is a complication associated with nebulized therapies, including CAYSTON. Reduction of 15% or more in forced expiratory volume in 1 second (FEV1) immediately following administration of study medication after pretreatment with a bronchodilator was observed in 3% of patients treated with CAYSTON.
5.3 Decreases in FEV1 After 28-Day Treatment Cycle
In clinical trials, patients with increases in FEV1 during a 28-day course of CAYSTON were sometimes treated for pulmonary exacerbations when FEV1 declined after the treatment period. Healthcare providers should consider a patient's baseline FEV1 measured prior to CAYSTON therapy and the presence of other symptoms when evaluating whether post-treatment changes in FEV1 are caused by a pulmonary exacerbation.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of drugs cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CAYSTON was evaluated in 344 patients from two placebo-controlled trials and one open-label follow-on trial. In controlled trials, 146 patients with CF received 75 mg CAYSTON 3 times a day for 28 days.
Table 1 displays adverse reactions reported in more than 5% of patients treated with CAYSTON 3 times a day in placebo-controlled trials. The listed adverse reactions occurred more frequently in CAYSTON-treated patients than in placebo-treated patients.
| Event (Preferred Term) | Placebo (N=160) n (%) | CAYSTON 75 mg 3 times a day (N=146) n (%) |
|---|---|---|
| Cough | 82 (51%) | 79 (54%) |
| Nasal congestion | 19 (12%) | 23 (16%) |
| Wheezing | 16 (10%) | 23 (16%) |
| Pharyngolaryngeal pain | 17 (11%) | 18 (12%) |
| Pyrexia | 9 (6%) | 19 (13%) |
| Chest discomfort | 10 (6%) | 11 (8%) |
| Abdominal Pain | 8 (5%) | 10 (7%) |
| Vomiting | 7 (4%) | 9 (6%) |
Adverse reactions that occurred in less than 5% of patients treated with CAYSTON were bronchospasm (3%) [see Warnings and Precautions (5.2)] and rash (2%).
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following possible adverse reactions have been identified during post-approval use of CAYSTON. Because these events have been reported voluntarily from a population of unknown size, estimates of frequency cannot be made.
MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS
Arthralgia, joint swelling
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data on CAYSTON use in pregnant women is insufficient to inform a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes; however, systemic absorption of aztreonam following inhaled administration is expected to be minimal [see Clinical Pharmacology (12.3)]. There are risks to the mother associated with cystic fibrosis in pregnancy (see Clinical Considerations). In animal reproduction studies with aztreonam for injection administered parenterally to pregnant rats and rabbits during organogenesis, there was no evidence of developmental toxicity. A peri/postnatal study in rats revealed no drug-induced changes in maternal, fetal, or neonatal parameters.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No reproductive toxicity studies have been conducted with CAYSTON. However, studies were conducted with aztreonam for injection. No evidence of developmental toxicity has been shown in studies with pregnant rats and rabbits that received parenteral doses of aztreonam during organogenesis of up to 1800 and 1200 mg/kg/day, respectively. In rats receiving aztreonam for injection during late gestation and lactation at up to 1800 mg/kg/day, no drug induced changes in maternal, fetal or neonatal parameters were observed. These animal reproduction and developmental toxicity studies used parenteral routes of administration that would provide systemic exposures significantly greater than the average peak plasma levels measured in humans following CAYSTON therapy.
8.2 Lactation
Risk Summary
Following intravenous administration of aztreonam for injection, aztreonam is excreted in human milk at concentrations that are less than one percent of those determined in simultaneously obtained maternal serum. Peak plasma concentrations of aztreonam following administration of CAYSTON (75 mg) are approximately 1% of peak concentrations observed following IV aztreonam (500 mg). Systemic absorption of aztreonam following inhaled administration is expected to be minimal [see Clinical Pharmacology (12.3)]. There are no data on the effects of aztreonam on the breastfed infant or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for CAYSTON and any potential adverse effects on the breastfed infant from CAYSTON or from the underlying maternal condition.
8.4 Pediatric Use
Patients 7 years and older were included in clinical trials with CAYSTON. Fifty-five patients under 18 years of age received CAYSTON in placebo-controlled trials. No dose adjustments were made for pediatric patients. Pyrexia was more commonly reported in pediatric patients than in adult patients. Safety and effectiveness in pediatric patients below the age of 7 years have not been established.
8.5 Geriatric Use
Clinical trials of CAYSTON did not include CAYSTON-treated patients aged 65 years of age and older to determine whether they respond differently from younger patients.
8.6 Use in Patients with Renal Impairment
Aztreonam is known to be excreted by the kidney. Placebo-controlled clinical trials with CAYSTON excluded patients with abnormal baseline renal function (defined as serum creatinine greater than 2 times the upper limit of normal range). Given the low systemic exposure of aztreonam following administration of CAYSTON, clinically relevant accumulation of aztreonam is unlikely to occur in patients with renal impairment. Therefore, CAYSTON may be administered to patients with mild, moderate and severe renal impairment with no dosage adjustment.
10 OVERDOSAGE
No overdoses have been reported with CAYSTON in clinical trials to date. In clinical trials, 225 mg doses of CAYSTON via inhalation were associated with higher rates of drug-related respiratory adverse reactions, particularly cough. Since the peak plasma concentration of aztreonam following administration of CAYSTON (75 mg) is approximately 0.6 mcg/mL, compared to a serum concentration of 54 mcg/mL following administration of aztreonam for injection (500 mg), no systemic safety issues associated with CAYSTON overdose are anticipated.
11 DESCRIPTION
A dose of CAYSTON consists of a 2 mL amber glass vial containing lyophilized aztreonam (75 mg) and lysine (46.7 mg), and a low-density polyethylene ampule containing 1 mL sterile diluent (0.17% sodium chloride). The reconstituted solution is for inhalation. The formulation contains no preservatives or arginine.
The active ingredient in CAYSTON is aztreonam, a monobactam antibacterial. The monobactams are structurally different from beta-lactam antibiotics (e.g., penicillins, cephalosporins, carbapenems) due to a monocyclic nucleus. This nucleus contains several side chains; sulfonic acid in the 1-position activates the nucleus, an aminothiazolyl oxime side chain in the 3-position confers specificity for aerobic Gram-negative bacteria including Pseudomonas spp., and a methyl group in the 4-position enhances beta-lactamase stability.
Aztreonam is designated chemically as (Z)-2-[[[(2-amino-4-thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid. The structural formula is presented below:

CAYSTON is a white to off-white powder. CAYSTON is sterile, hygroscopic, and light sensitive. Once reconstituted with the supplied diluent, the pH range is 4.5 to 6.0.
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Sputum Concentrations
Sputum aztreonam concentrations exhibited considerable variability between patients receiving CAYSTON (75 mg) in clinical trials. The mean sputum concentration 10 minutes following the first dose of CAYSTON (n = 195 patients with CF) was 726 mcg/g. Mean sputum concentrations of aztreonam in patients receiving CAYSTON 3 times a day for 28 days were 984 mcg/g, 793 mcg/g, and 715 mcg/g 10 minutes after dose administration on Days 0, 14, and 28, respectively, indicating no accumulation of aztreonam in sputum.
Plasma Concentrations
Plasma aztreonam concentrations exhibited considerable variability between patients receiving CAYSTON (75 mg) in the clinical trials. The mean plasma concentration one hour following the first dose of CAYSTON (at approximately the peak plasma concentration) was 0.59 mcg/mL. Mean peak plasma concentrations in patients receiving CAYSTON 3 times a day for 28 days were 0.55 mcg/mL, 0.67 mcg/mL, and 0.65 mcg/mL on Days 0, 14, and 28, respectively, indicating no systemic accumulation of aztreonam. In contrast, the serum concentration of aztreonam following administration of aztreonam for injection (500 mg) is approximately 54 mcg/mL.
Absorption
Evaluation of plasma and urine aztreonam concentrations following administration of CAYSTON indicates low systemic absorption of aztreonam. Approximately 10% of the total CAYSTON dose is excreted in the urine as unchanged drug, as compared to 60–65% following intravenous administration of aztreonam for injection.
Distribution
The protein binding of aztreonam in plasma is approximately 77% within the clinical dose range of concentrations achieved following CAYSTON administration.
Metabolism
Following intramuscular administration of aztreonam for injection 500 mg every 8 hours for 7 days, approximately 6% of the dose was excreted as a microbiologically inactive open β-lactam ring hydrolysis product in an 8-hour urine collection on the last day of multiple dosing.
Excretion
The elimination half-life of aztreonam from plasma is approximately 2.1 hours following administration of CAYSTON to adult patients with CF, similar to what has been reported for aztreonam for injection. Approximately 10% of the total CAYSTON dose is excreted in the urine as unchanged drug. Systemically absorbed aztreonam is eliminated about equally by active tubular secretion and glomerular filtration. Following administration of a single intravenous dose of radiolabeled aztreonam for injection, about 12% of the dose was recovered in the feces.
12.4 Microbiology
Mechanism of Action
Aztreonam exhibits activity in vitro against Gram-negative aerobic pathogens including P. aeruginosa. Aztreonam binds to penicillin-binding proteins of susceptible bacteria, which leads to inhibition of bacterial cell wall synthesis and death of the cell. Aztreonam activity is not decreased in the presence of CF lung secretions.
Susceptibility Testing
A single sputum sample from a patient with CF may contain multiple morphotypes of P. aeruginosa and each morphotype may have a different level of in vitro susceptibility to aztreonam. There are no in vitro susceptibility test interpretive criteria for isolates of P. aeruginosa obtained from the sputum of CF patients.1
Development of Resistance
No changes in the susceptibility of P. aeruginosa to aztreonam were observed following a 28-day course of CAYSTON in the placebo-controlled trials.
Cross-Resistance
No cross-resistance to other classes of antibiotics, including aminoglycosides, quinolones, and beta-lactams, was observed following a 28-day course of CAYSTON in the Phase 3 placebo-controlled trials or in an open-label follow-on trial of up to nine 28-day courses of 75 mg CAYSTON 3 times a day.
Other
No trends in the treatment-emergent isolation of other bacterial respiratory pathogens (Burkholderia cepacia, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, and Staphylococcus aureus) were observed in clinical trials. There was a slight increase in the isolation of Candida spp. following up to nine 28-day courses of CAYSTON therapy.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 104-week rat inhalation toxicology study to assess the carcinogenic potential of aztreonam demonstrated no drug-related increase in the incidence of tumors. Rats were exposed to aerosolized aztreonam for up to 4 hours per day. Peak plasma levels of aztreonam averaging approximately 6.8 mcg/mL were measured in rats at the highest dose level. This is approximately 12-fold higher than the average peak plasma level measured in humans following CAYSTON therapy.
Genetic toxicology studies performed in vitro demonstrated that aztreonam did not induce structural chromosome aberrations in CHO cells and did not induce mutations at the TK locus in mouse lymphoma L5178Y TK+/- cells. In vivo, aztreonam was not clastogenic in mouse bone marrow cells.
Aztreonam did not impair the fertility of rats when administered parenterally at doses up to 2400 mg/kg/day that would provide systemic exposures significantly higher than peak plasma levels measured in humans following CAYSTON therapy.
14 CLINICAL STUDIES
CAYSTON was evaluated over a period of 28 days of treatment in a randomized, double-blind, placebo-controlled, multicenter trial that enrolled patients with CF and P. aeruginosa. This trial was designed to evaluate improvement in respiratory symptoms. Patients 7 years of age and older and with FEV1 of 25% to 75% predicted were enrolled. All patients received CAYSTON or placebo on an outpatient basis administered with the Altera Nebulizer System. All patients were required to take a dose of an inhaled bronchodilator (beta-agonist) prior to taking a dose of CAYSTON or placebo. Patients were receiving standard care for CF, including drugs for obstructive airway diseases.
The trial enrolled 164 patients with CF and P. aeruginosa. The mean age was 30 years, and the mean baseline FEV1 % predicted was 55%; 43% were females and 96% were Caucasian. These patients were randomized in a 1:1 ratio to receive either CAYSTON (75 mg) or volume-matched placebo administered by inhalation 3 times a day for 28 days. Patients were required to have been off antibiotics for at least 28 days before treatment with study drug. The primary efficacy endpoint was improvement in respiratory symptoms on the last day of treatment with CAYSTON or placebo. Respiratory symptoms were also assessed two weeks after the completion of treatment with CAYSTON or placebo. Changes in respiratory symptoms were assessed using a questionnaire that asks patients to report on symptoms like cough, wheezing, and sputum production.
Improvement in respiratory symptoms was noted for CAYSTON-treated patients relative to placebo-treated patients on the last day of drug treatment. Statistically significant improvements were seen in both adult and pediatric patients but were substantially smaller in adult patients. Two weeks after completion of treatment, a difference in respiratory symptoms between treatment groups was still present, though the difference was smaller.
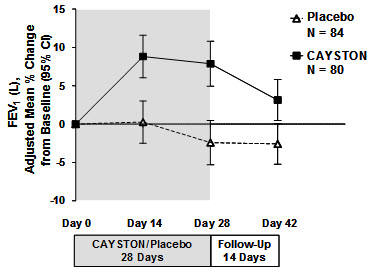
Pulmonary function, as measured by FEV1 (L), increased from baseline in patients treated with CAYSTON (see Figure 1). The treatment difference at Day 28 between CAYSTON-treated and placebo-treated patients for percent change in FEV1 (L) was statistically significant at 10% (95% CI: 6%, 14%). Improvements in FEV1 were comparable between adult and pediatric patients. Two weeks after completion of drug treatment, the difference in FEV1 between CAYSTON and placebo groups had decreased to 6% (95% CI: 2%, 9%).
Figure 1 Adjusted Mean Percent Change in FEV1 from Baseline to Study End (Days 0–42)
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
Each kit for a 28-day course of CAYSTON contains 84 sterile vials of CAYSTON and 88 ampules of sterile diluent packed in 2 cartons, each carton containing a 14-day supply. The four additional diluent ampules are provided in case of spillage.
| Package Configuration | Dosage Strength | NDC |
|---|---|---|
| 28-Day Kit | 75 mg | 61958-0901-1 |
CAYSTON vials and diluent ampules should be stored in the refrigerator at 2 °C to 8 °C (36 °F to 46 °F) until needed. Once removed from the refrigerator, CAYSTON and diluent may be stored at room temperature (up to 25 °C/77 °F) for up to 28 days. Do not separate the CAYSTON vials from the diluent ampules. CAYSTON should be protected from light.
Do not use CAYSTON if it has been stored at room temperature for more than 28 days. Do not use CAYSTON beyond the expiration date stamped on the vial. Do not use diluent beyond the expiration date embossed on the ampule.
CAYSTON should be used immediately upon reconstitution. Do not reconstitute more than one dose at a time.
Do not use diluent or reconstituted CAYSTON if it is cloudy or if there are particles in the solution.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Allergic Reactions
Advise patients to tell their healthcare provider immediately if they believe they are experiencing new or worsening symptoms or believe they are having an allergic reaction to CAYSTON [see Warnings and Precautions (5.1)].
Development of Drug-Resistant Bacteria
Counsel patients that antibacterial drugs including CAYSTON should only be used to treat bacterial infections. They do not treat viral infection (e.g., the common cold). When CAYSTON is prescribed to treat a bacterial infection, inform patients that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by CAYSTON or other antibacterial drugs in the future [see Warnings and Precautions (5.4)].
Reconstitution and Administration
Advise patients that:
- CAYSTON is for inhalation use only and should only be administered using the Altera Nebulizer System.
- CAYSTON should only be reconstituted with the provided diluent. Instruct patients not to mix other drugs with CAYSTON in the Altera Nebulizer System.
Advise patients to:
- use a bronchodilator prior to administration of CAYSTON.
- complete the full 28-day course of CAYSTON even if they are feeling better.
Advise patients taking several inhaled medications to use the medications in the following order: bronchodilator, mucolytics, and lastly, CAYSTON.
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
| This Patient Information has been approved by the U.S. Food and Drug Administration | Rev November 2019 |
| PATIENT INFORMATION CAYSTON® (kay-stun) (aztreonam for inhalation solution) for oral inhalation use |
|
| What is CAYSTON?
CAYSTON is a prescription medicine that is used to improve breathing symptoms in people with cystic fibrosis (CF) who have a lung infection caused by a bacterium called Pseudomonas aeruginosa. CAYSTON contains an antibacterial medicine called aztreonam. |
|
|
|
| It is not known if CAYSTON is safe and effective: | |
|
|
| Do not take CAYSTON if you are allergic to aztreonam, or any of the ingredients in CAYSTON. See the end of this Patient Information for a complete list of ingredients in CAYSTON. |
|
| Before you take CAYSTON, tell your healthcare provider about all of your medical conditions, including if you: | |
|
|
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how CAYSTON works. |
|
| How should I take CAYSTON? | |
|
|
| What are the possible side effects of CAYSTON? CAYSTON may cause serious side effects, including: |
|
|
|
| The most common side effects of CAYSTON include: | |
|
|
| Other possible side effects of CAYSTON include swelling or pain in joints. Tell your healthcare provider if you have any new or worsening symptoms while taking CAYSTON. Tell your healthcare provider about any side effect that bothers you or that does not go away. |
|
| These are not all the possible side effects of CAYSTON. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
| How should I store CAYSTON? | |
|
|
| Keep CAYSTON and all medicines out of the reach of children. | |
| General information about the safe and effective use of CAYSTON
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CAYSTON for a condition for which it was not prescribed. Do not give CAYSTON to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about CAYSTON that is written for health professionals. |
|
| What are the ingredients in CAYSTON? Active ingredient: aztreonam Inactive ingredient: lysine, sodium chloride (diluent) Manufactured and distributed by: Gilead Sciences, Inc. Foster City, CA 94404 CAYSTON is a registered trademark of Gilead Sciences, Inc. ©2019 Gilead Sciences, Inc. All rights reserved. 50-814-GS-004 For more information, call 1-877-7CAYSTON (1-877-722-9786). |
|
PATIENT INSTRUCTIONS FOR USE
CAYSTON®
(aztreonam for inhalation solution)
for oral inhalation use
Be sure that you read, understand and follow the Patient Instructions for Use below for the right way to take CAYSTON. If you have any questions, ask your healthcare provider or pharmacist.
You will need the following supplies (Figure 1):
- 1 amber colored CAYSTON vial covered by a metal seal with a blue cap
- 1 ampule of saline (diluent)
- Altera Nebulizer System

Figure 1
Check to make sure that your Altera Nebulizer System works properly before starting your treatment with CAYSTON. See the manufacturer's instructions for use that comes with your Altera Nebulizer System. This should have complete information about how to put together (assemble), prepare, use, and care for your Altera Nebulizer System.
Preparing your CAYSTON for Inhalation
- Step 1. Mix (reconstitute) CAYSTON with the saline only when ready to take a dose. Take 1 amber vial of CAYSTON and 1 ampule of saline from the carton. Separate the saline ampules by gently pulling apart.
- Step 2. Look at the ampule of saline. If it looks cloudy do not use it. Throw away this ampule and get another ampule of saline.
- Step 3. Gently tap the vial so that the powder settles to the bottom of the vial. This helps you get the proper dose of medicine. Follow Step A to Step D in Figure 2 below to open the vial:
|
Step A: With the blue cap tab facing toward you, place the vial on a flat surface. Using one hand to hold the vial steady, use the other hand to slowly flip up the blue cap. |
Step B: Pull the blue cap down to a flat (horizontal) position (where the bottom of the blue cap faces up), to prepare the metal seal for removal. Do not completely tear through the metal seal. |
|
Step C: While continuing to hold the vial steady with one hand, use the other hand to slowly pull the blue cap in a counterclockwise direction. Do not twist the blue cap. |
Step D: When the metal seal opens, continue to slowly pull the blue cap in a counterclockwise direction until the metal seal is completely removed. |
| Figure 2 | |
- Step 4. Safely throw away (dispose of) the metal seal in household garbage. Carefully remove (but do not yet discard) the rubber stopper.
-
Step 5. Open the ampule of saline by twisting off the tip. Squeeze out the contents completely into the vial (Figure 3). Next, close the vial with the rubber stopper and gently swirl the vial until the powder has completely dissolved and the liquid is clear.

Figure 3
- Step 6. After mixing CAYSTON with the saline, check to make sure the diluted medicine is clear. If it is cloudy or has particles in it, do not use this medicine. Throw away this dose of medicine and start over again with a new vial of CAYSTON and a new ampule of saline.
- Step 7. Use CAYSTON right away after you mix with the saline.
Taking your CAYSTON Treatment
See the manufacturer's instructions for use that comes with your Altera Nebulizer System for complete instructions on taking a treatment, and how to clean and disinfect your Altera Nebulizer Handset.
- Step 8. Make sure the handset is on a flat, stable surface.
-
Step 9. Remove the rubber stopper from the vial, then pour all of the mixed CAYSTON and saline into the Medication Reservoir of the handset (Figure 4). Be sure to completely empty the vial, gently tapping the vial against the side of the Medication Reservoir if necessary. Close the Medication Reservoir (Figure 5).

Figure 4

Figure 5
-
Step 10. Begin your treatment by sitting in a relaxed, upright position. Hold the handset level, and place the Mouthpiece in your mouth. Close your lips around the Mouthpiece (Figure 6).

Figure 6
- Step 11. Breathe in and out normally (inhale and exhale) through the Mouthpiece. Avoid breathing through your nose. Continue to inhale and exhale comfortably until the treatment is finished.
- Step 12. The empty vial, stopper and saline ampule should be disposed of in household garbage upon completion of dosing.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
SPL UNCLASSIFIED SECTION
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Cayston 28 Day Carton - Representative Label
NDC 61958-0901-1
GILEAD
Cayston®
(aztreonam for
inhalation solution)
75 mg/vial
For Oral Inhalation Only
Store Refrigerated, 2 °C to 8 °C (36 °F to 46 °F)
Contains:
84 Single-Use Vials of Aztreonam for Inhalation Solution
88 Diluent Ampules of Sodium Chloride 0.17%, 1 mL
(4 extra ampules provided in case of spillage)
For use only with the Altera® Nebulizer System
28-Day Supply
Rx only

INGREDIENTS AND APPEARANCE
| CAYSTON
aztreonam kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Gilead Sciences, Inc. (185049848) |



