Search by Drug Name or NDC
NDC 63736-0375-01 Americaine 5.6 g/28g Details
Americaine 5.6 g/28g
Americaine is a TOPICAL OINTMENT in the HUMAN OTC DRUG category. It is labeled and distributed by Insight Pharmaceuticals LLC. The primary component is BENZOCAINE.
Product Information
| NDC | 63736-0375 |
|---|---|
| Product ID | 63736-375_272a1b21-943a-4bbc-acfd-2a5e3433ef05 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Americaine |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Benzocaine |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | OINTMENT |
| Route | TOPICAL |
| Active Ingredient Strength | 5.6 |
| Active Ingredient Units | g/28g |
| Substance Name | BENZOCAINE |
| Labeler Name | Insight Pharmaceuticals LLC |
| Pharmaceutical Class | Allergens [CS], Cell-mediated Immunity [PE], Increased Histamine Release [PE], Standardized Chemical Allergen [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part346 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
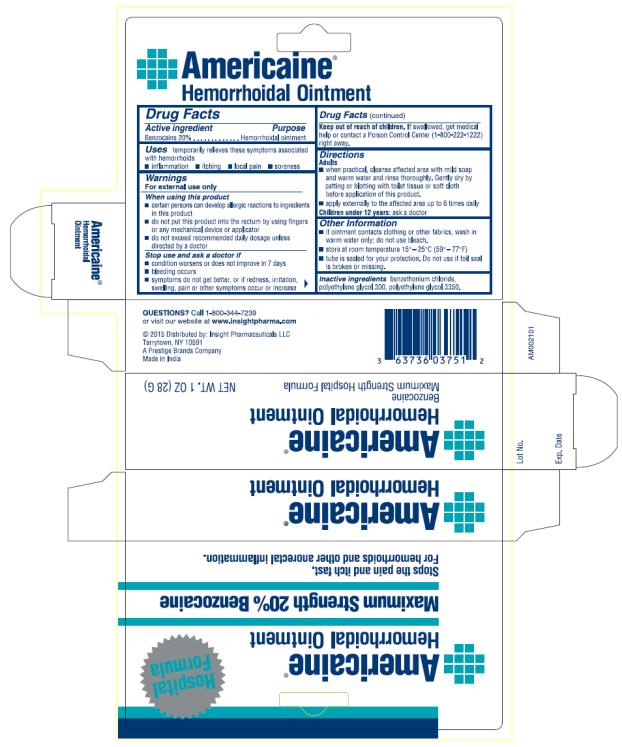
NDC 63736-0375-01 (63736037501)
| NDC Package Code | 63736-375-01 |
|---|---|
| Billing NDC | 63736037501 |
| Package | 1 TUBE in 1 BOX (63736-375-01) / 28 g in 1 TUBE |
| Marketing Start Date | 2009-07-10 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL d13c022f-2f91-40b4-bd66-0cfc0e147a5f Details
Uses
Warnings
For external use only
When using this product
- certain persons can develop allergic reactions to ingredients in this product
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- do not exceed dosage unless directed by a doctor
Directions
Other information
QUESTIONS?
INGREDIENTS AND APPEARANCE
| AMERICAINE
benzocaine ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Insight Pharmaceuticals LLC (055665422) |
Revised: 1/2020
Document Id: 272a1b21-943a-4bbc-acfd-2a5e3433ef05
Set id: d13c022f-2f91-40b4-bd66-0cfc0e147a5f
Version: 4
Effective Time: 20200114