Search by Drug Name or NDC
NDC 63824-0768-16 Cepacol 15; 2.3 mg/1; mg/1 Details
Cepacol 15; 2.3 mg/1; mg/1
Cepacol is a ORAL LOZENGE in the HUMAN OTC DRUG category. It is labeled and distributed by RB Health (US) LLC. The primary component is BENZOCAINE; MENTHOL, UNSPECIFIED FORM.
Product Information
| NDC | 63824-0768 |
|---|---|
| Product ID | 63824-768_05eb3c99-8580-4f4d-9174-5c49fe3f1d50 |
| Associated GPIs | 88359902154792 |
| GCN Sequence Number | 079623 |
| GCN Sequence Number Description | benzocaine/menthol LOZENGE 15MG-2.3MG MUCOUS MEM |
| HIC3 | H0A |
| HIC3 Description | LOCAL ANESTHETICS |
| GCN | 46156 |
| HICL Sequence Number | 001675 |
| HICL Sequence Number Description | BENZOCAINE/MENTHOL |
| Brand/Generic | Brand |
| Proprietary Name | Cepacol |
| Proprietary Name Suffix | Extra Strength Sore Throat Tangerine |
| Non-Proprietary Name | Benzocaine and Menthol, Unspecified Form |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | LOZENGE |
| Route | ORAL |
| Active Ingredient Strength | 15; 2.3 |
| Active Ingredient Units | mg/1; mg/1 |
| Substance Name | BENZOCAINE; MENTHOL, UNSPECIFIED FORM |
| Labeler Name | RB Health (US) LLC |
| Pharmaceutical Class | Allergens [CS], Cell-mediated Immunity [PE], Increased Histamine Release [PE], Standardized Chemical Allergen [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part356 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
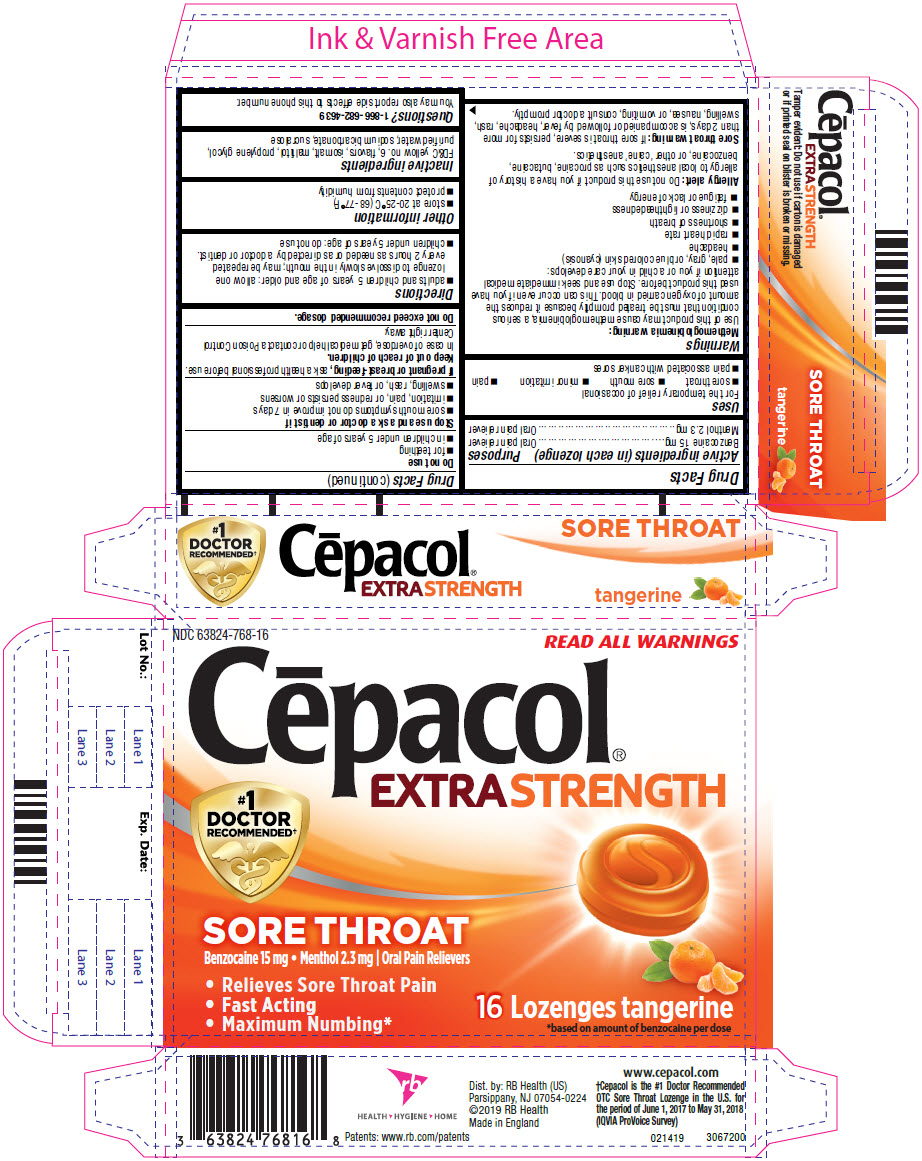
NDC 63824-0768-16 (63824076816)
| NDC Package Code | 63824-768-16 |
|---|---|
| Billing NDC | 63824076816 |
| Package | 2 BLISTER PACK in 1 CARTON (63824-768-16) / 8 LOZENGE in 1 BLISTER PACK |
| Marketing Start Date | 2015-07-15 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b06cc02e-5385-4d21-89b8-7a4900ce5531 Details
SPL UNCLASSIFIED SECTION
Uses
Warnings
Methemoglobinemia warning
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other 'caine' anesthetics.
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly.
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- swelling, rash, or fever develops
Directions
Inactive ingredients
PRINCIPAL DISPLAY PANEL - 16 Lozenge Blister Pack Carton
INGREDIENTS AND APPEARANCE
| CEPACOL
EXTRA STRENGTH SORE THROAT TANGERINE
benzocaine and menthol, unspecified form lozenge |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |