Search by Drug Name or NDC
NDC 65808-0326-01 Real Relief 8; 8; 8; 8; 8; 8; 8; 8 [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1 Details
Real Relief 8; 8; 8; 8; 8; 8; 8; 8 [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1
Real Relief is a ORAL TABLET in the HUMAN OTC DRUG category. It is labeled and distributed by GMP Laboratories of America, Inc.. The primary component is ARNICA MONTANA; BRYONIA ALBA ROOT; CAUSTICUM; CINCHONA OFFICINALIS BARK; GOLDENSEAL; POTASSIUM BROMIDE; SOLANUM DULCAMARA TOP; SULFUR.
Product Information
| NDC | 65808-0326 |
|---|---|
| Product ID | 65808-326_b7a21773-caf1-2960-e053-2995a90ada31 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Real Relief |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Arnica montana, Bryonia, Causticum, Cinchona officinalis, Dulcamara, Hydrastis canadensis, Kali bromatum, Sulphur |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 8; 8; 8; 8; 8; 8; 8; 8 |
| Active Ingredient Units | [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1 |
| Substance Name | ARNICA MONTANA; BRYONIA ALBA ROOT; CAUSTICUM; CINCHONA OFFICINALIS BARK; GOLDENSEAL; POTASSIUM BROMIDE; SOLANUM DULCAMARA TOP; SULFUR |
| Labeler Name | GMP Laboratories of America, Inc. |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | UNAPPROVED HOMEOPATHIC |
| Application Number | n/a |
| Listing Certified Through | 2024-12-31 |
Package
Package Images




NDC 65808-0326-01 (65808032601)
| NDC Package Code | 65808-326-01 |
|---|---|
| Billing NDC | 65808032601 |
| Package | 1 BOTTLE in 1 CARTON (65808-326-01) / 90 TABLET in 1 BOTTLE |
| Marketing Start Date | 2020-01-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b7a1fb57-3f47-7cf2-e053-2a95a90a312c Details
SPL UNCLASSIFIED SECTION
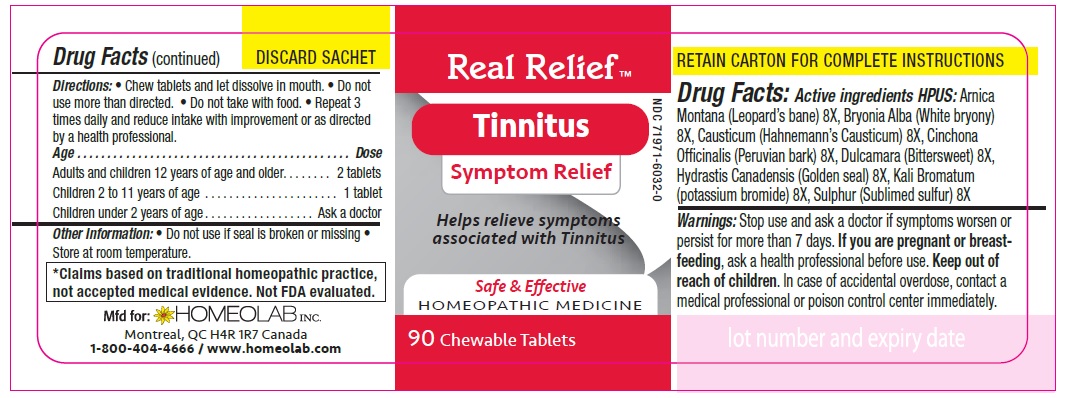
Active Ingredients HPUS:
Arnica montana (Leopard's bane) 8[hp-X]
Bryonia (White bryony) 8 [hp-X]
Causticum (Hahnmann's causticum) 8 [hp-X]
Cinchona officinalis (Peruvian bark) 8 [hp-X]
Dulcamara (Bittersweet) 8 [hp-X]
Hydrastis canadensis (Golden seal) 8 [hp-X]
Kali bromatum (Potassium bromide) 8 [hp-X]
Sulphur (Sublimed sulfur) 8 [hp-X]
SPL UNCLASSIFIED SECTION
Purpose
Homeopathic remedy helps relieve symptoms of tinnitus:
- Rigging and buzzing in the ears
- Ear pain & pressure
- Headache
The letters 'HPUS' indicate that the components in this product are officially monographed in the Homoeopathic Pharmacopoeia of the United States.
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
Directions
Chew tablets and let dissolve in mouth.
Do not use more than directed.
Do not take with food.
Repeat 3 times daily and reduce intake with improvement or as directed by a health professional
Age…………………………………………………………........ Dose
Adults and Children 12 years of age and older………...... 2 tablets
Children 2 to 11 years of age…...……………………............ 1 tablet
Children under 2 years of age……………………................. Ask a doctor
SPL UNCLASSIFIED SECTION
INGREDIENTS AND APPEARANCE
| REAL RELIEF
arnica montana, bryonia, causticum, cinchona officinalis, dulcamara, hydrastis canadensis, kali bromatum, sulphur tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - GMP Laboratories of America, Inc. (876754375) |