Search by Drug Name or NDC
NDC 65857-0505-10 Kit for the Preparation of Technetium Tc 99m Medronate 20 mg/1 Details
Kit for the Preparation of Technetium Tc 99m Medronate 20 mg/1
Kit for the Preparation of Technetium Tc 99m Medronate is a INTRAVENOUS INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Cardinal Health 414, LLC. The primary component is TECHNETIUM TC-99M MEDRONATE.
Product Information
| NDC | 65857-0505 |
|---|---|
| Product ID | 65857-505_46ba888d-5102-40c6-b550-4c18e55f1f85 |
| Associated GPIs | |
| GCN Sequence Number | 066236 |
| GCN Sequence Number Description | kit for Tc-99m/medronate sod VIAL 20 MG INTRAVEN |
| HIC3 | Z9D |
| HIC3 Description | DIAGNOSTIC PREPARATIONS,MISCELLANEOUS |
| GCN | 28467 |
| HICL Sequence Number | 024559 |
| HICL Sequence Number Description | KIT FOR PREPARATION OF TC-99M/MEDRONATE SODIUM |
| Brand/Generic | Generic |
| Proprietary Name | Kit for the Preparation of Technetium Tc 99m Medronate |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | technetium tc 99m medronate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 20 |
| Active Ingredient Units | mg/1 |
| Substance Name | TECHNETIUM TC-99M MEDRONATE |
| Labeler Name | Cardinal Health 414, LLC |
| Pharmaceutical Class | Radioactive Diagnostic Agent [EPC], Radiopharmaceutical Activity [MoA] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA018107 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 65857-0505-10 (65857050510)
| NDC Package Code | 65857-505-10 |
|---|---|
| Billing NDC | 65857050510 |
| Package | 10 INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION in 1 CARTON (65857-505-10) |
| Marketing Start Date | 2020-12-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 54608807-4df6-49ba-b065-59a19ef224c4 Details
DESCRIPTION
Each reaction vial contains a sterile, nonpyrogenic, nonradioactive lyophilized mixture of 20 mg medronic acid, 1 mg ascorbic acid, 0.13 mg (minimum) stannous fluoride, SnF2 and 0.38 mg total tin, maximum (as stannous fluoride, SnF2).
The pH is adjusted with sodium hydroxide or hydrochloric acid to 6.5 (6.3 to 6.7) prior to lyophilization. The vial does not contain a preservative. The contents of the vial are lyophilized and sealed under nitrogen at the time of manufacture.
The pH of the reconstituted product is 5.4 to 6.8.
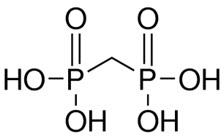
The structure of medronic acid is given below:
The precise structure of technetium Tc 99m medronate is unknown at this time.
When sterile, pyrogen-free sodium pertechnetate Tc 99m injection is added to the vial, the diagnostic agent technetium Tc 99m medronate is formed for administration by intravenous injection.
PHYSICAL CHARACTERISTICS
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.1 The principal photon that is useful for detection and imaging studies is shown in Table 1.
| 1 Kocher, David C: Radioactive Decay Data Tables, DOE/TlC-11026, 108, 1981 | ||
|
Principal Radiation Emission Data |
||
|
Radiation |
Mean % per |
Mean Energy |
|
Gamma-2 |
89.07 |
140.5 |
External Radiation
The specific gamma ray constant for Tc 99m is 0.78 R/ hour-millicurie at 1 cm. The first half-value layer is 0.017 cm lead (Pb). A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. To facilitate control of the radiation exposure from millicurie amounts of this radionuclide, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000.
|
Radiation Attenuation by Lead Shielding |
|
|
Shield Thickness |
Coefficient |
|
0.017 |
0.5 |
|
0.08 |
10-1 |
|
0.16 |
10-2 |
|
0.25 |
10-3 |
|
0.33 |
10-4 |
To correct for physical decay of technetium Tc 99m, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
| * Calibration Time | |||||
|
Physical Decay Chart: Tc 99m, half-life 6.02 hours |
|||||
|
Hours |
Fraction |
Hours |
Fraction |
||
|
0* |
100.0 |
7 |
0.447 |
||
|
1 |
0.891 |
8 |
0.398 |
||
|
2 |
0.794 |
9 |
0.355 |
||
|
3 |
0.708 |
10 |
0.316 |
||
|
4 |
0.631 |
11 |
0.282 |
||
|
5 |
0.562 |
12 |
0.251 |
||
|
6 |
0.501 | ||||
CLINICAL PHARMACOLOGY
During the initial 24 hours following intravenous injection of technetium Tc 99m medronate, about 50 percent of the dose is retained in the skeleton, and about 50 percent is excreted in the urine. A minimum amount of uptake has been observed in soft-tissue organs, most notably the kidneys.
Clearance of radioactivity from the blood is quite rapid, with about 10 percent of the injected dose remaining at one hour, and less than 5 and 2 percent at two and four hours, respectively. The rapid blood clearance (T ½: 38 to 75 minutes) provides bone to non-osseous tissue ratios favoring early imaging.
Following intravenous administration of technetium Tc 99m medronate, skeletal uptake occurs as a function of blood flow to bone and bone efficiency in extracting the complex. Bone mineral crystals are generally considered to be hydroxyapatite, and the complex appears to have an affinity for the hydroxyapatite crystals in bone.
Deposition of radioactivity in bone is rapid and appears to be related to osteogenic activity as well as the aforementioned skeletal blood perfusion. Skeletal uptake is bilaterally uniform, with larger concentrations in the axial structure and in the long bones. Increased accumulation of radioactivity may be seen, generally, in any bone disease state in which there is increased osteogenesis or a localized increase in osseous blood perfusion; consequently, bone imaging agents generally are not effective in detecting chronic bone diseases.
INDICATIONS AND USAGE
WARNINGS
This class of compounds is known to complex cations such as calcium. Particular caution should be used with patients who have or who may be predisposed to hypocalcemia (i.e., alkalosis).
Preliminary reports indicate interference with brain scans using sodium pertechnetate Tc 99m injection which have been preceded by a bone scan using an agent containing stannous ions. This interference may result in false-positive or false-negative brain scans. It is recommended, where feasible, that brain scans precede bone imaging procedures.
PRECAUTIONS
General
The contents of the kit before preparation are not radioactive. However, after the sodium pertechnetate Tc 99m injection is added, adequate shielding of the final preparation must be maintained.
Contents of the reaction vial are intended only for use in the preparation of technetium Tc 99m medronate and are NOT to be administered directly to the patient.
Technetium Tc 99m medronate as well as other radioactive drugs, must be handled with care. Once sodium pertechnetate Tc 99m is added to the vial, appropriate safety measures should be used to minimize external radiation to clinical occupational personnel. Care should also be taken to minimize radiation exposure to patients in a manner consistent with proper patient management.
To minimize the radiation dose to the bladder, the patient should be encouraged to drink fluids and to void immediately before the examination and as often thereafter as possible for the next four to six hours.
Technetium Tc 99m medronate should be formulated within six (6) hours prior to clinical use. Optimal imaging results are obtained one to four hours after administration. Technetium Tc 99m medronate injection should be discarded six hours after reconstitution. The solution should not be used if cloudy.
The components of the kit are sterile and pyrogen-free. It is essential to follow directions carefully and to adhere to strict aseptic procedures during preparation.
The technetium Tc 99m labeling reactions involved in preparing the agent depend on maintaining the stannous ion in the reduced state. Any oxidant present in the sodium pertechnetate Tc 99m supply may, thus, adversely affect the quality of the radiopharmaceutical. Hence, sodium pertechnetate Tc 99m containing oxidants should not be employed.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential or whether technetium Tc 99m medronate affects fertility in males or females.
Pregnancy
Animal reproduction studies have not been conducted with technetium Tc 99m medronate. It is also not known whether technetium Tc 99m medronate can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Technetium Tc 99m medronate should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Several adverse reactions due to technetium Tc 99m medronate have been reported. These were usually hypersensitivity reactions characterized by itching, various skin rashes, hypotension, chills, nausea and vomiting. There have also been rare cases of dizziness and asthenia associated with the use of technetium Tc 99m medronate.
DOSAGE AND ADMINISTRATION
After preparation with oxidant-free sodium pertechnetate Tc 99m injection the suggested dose range of technetium Tc 99m medronate injection in the average patient (70 kg) is 370 MBq to 740 MBq (10 mCi to 20 mCi) given intravenously. Imaging post injection is optimal at 1 to 4 hours.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Shielding should be utilized when preparing the technetium Tc 99m medronate injection.
Radiation Dosimetry
The effective half-life was assumed to be the physical half-life for all calculated values. The estimated radiation absorbed doses to an average patient (70 kg) from an intravenous injection of a maximum dose of 740 MBq (20 mCi) of technetium Tc 99m medronate are shown in Table 4.
|
Absorbed Radiation Dose |
|||
|
Organ |
mGy/740 MBq |
rads/20 mCi |
|
|
Total Body |
1.3 |
0.13 |
|
|
Bone Total |
7.0 |
0.70 |
|
|
Red Marrow |
5.6 |
0.56 |
|
|
Kidneys |
8.0 |
0.80 |
|
|
Liver |
0.6 |
0.06 |
|
|
Bladder Wall |
2-hr. void |
26.0 |
2.60 |
|
4.8-hr. void |
62.0 |
6.20 |
|
|
Ovaries |
2-hr. void |
2.4 |
0.24 |
|
4.8-hr. void |
3.4 |
0.34 |
|
|
Testes |
2-hr. void |
1.6 |
0.16 |
|
4.8-hr. void |
2.2 |
0.22 |
|
Method of Calculation: “S” Absorbed Dose per Unit Cumulated Activity for Selected Radionuclides and Organs, MIRD Pamphlet No. 11, 1975
HOW SUPPLIED
Kit for the Preparation of Technetium Tc 99m Medronate is supplied as kits of 10 reaction vials (NDC 65857-505-10).
Each reaction vial contains a sterile, nonpyrogenic, nonradioactive lyophilized mixture of 20 mg medronic acid, 1 mg ascorbic acid, 0.13 mg (minimum) stannous fluoride, SnF2 and 0.38 mg total tin, maximum (as stannous fluoride, SnF2).
The pH is adjusted with sodium hydroxide or hydrochloric acid prior to lyophilization. The vial does not contain a preservative. The contents of the vial are lyophilized and sealed under nitrogen at the time of manufacture. The pH of the reconstituted product is 5.4 to 6.8.
Kit Contents
10 sterile reaction vials
20 pressure-sensitive labels for technetium Tc 99m medronate
1 package insert
Storage
Store the product as supplied at 20° to 25°C (68° to 77°F). After reconstitution store refrigerated at 2° to 8°C (36° to 46°F) (see DOSAGE AND ADMINISTRATION).
Do not use and discard radiolabeled technetium Tc 99m medronate 6 hours after reconstitution.
DIRECTIONS FOR PREPARATION OF TECHNETIUM Tc 99m MEDRONATE
Procedural Precautions
The lyophilized powder in the reaction vial is sterile and nonpyrogenic and does not contain a preservative. Aseptic procedure and shielded syringes should be used when adding the pertechnetate eluate to the reaction vial and for withdrawal and administration of the dose of the finished medronate agent. Waterproof gloves should be worn to prevent the possibility of radioactive contamination of the hands.
If sodium pertechnetate Tc 99m must be diluted prior to injection into the reaction vial, only preservative-free 0.9% Sodium Chloride Injection USP should be used.
Preparation
Preparation of technetium Tc 99m medronate is done by the following aseptic procedure:
- 1.
- Waterproof gloves should be worn during the preparation procedure.
- 2.
- Place reaction vial in an appropriate lead shield.
- 3.
- Remove the central disc from the reaction vial and swab the rubber closure of the reaction vial with a germicide.
- 4.
- With a sterile syringe, aseptically obtain 0.5 mL to 5 mL [up to 18,500 MBq (500 mCi)] of a suitable, oxidant-free, sterile nonpyrogenic sodium pertechnetate.
- 5.
- Aseptically add the sodium pertechnetate Tc 99m solution to the vial.
- 6.
- Secure the lead shield cover. Swirl the vial for one minute and let stand for one to two minutes.
- 7.
- Record the date and time of preparation on pressure-sensitive label.
- 8.
- Affix pressure-sensitive label to shield.
- 9.
- Examine vial contents. If the solution is not clear and free of particulate matter and discoloration on visual inspection, it should not be used.
- 10.
- Measure the radioactivity using a suitable calibration system and record on the shield label prior to patient administration.
- 11.
- Aseptically withdraw material for use within six (6) hours of preparation. For optimum results, this time should be minimized. The vial contains no bacteriostatic preservative.
The U.S. Nuclear Regulatory Commission has approved this reagent kit for distribution to persons licensed to use byproduct material identified in §35.200 of 10 CFR Part 35, to persons who hold an equivalent license issued by an Agreement State, and, outside the United States, to persons authorized by the appropriate authority.
Distributed by:
Cardinal Health 414, LLC
Dublin, OH 43017
Revised April 2020
PRINCIPAL DISPLAY PANEL - Carton Label
DIAGNOSTIC
NDC 65857-505-10
Kit for the Preparation of Technetium Tc 99m Medronate
Rx only • Store the product at 20° to 25°C (68° to 77°F) • For Intravenous Use after radiolabeling with
sodium pertechnetate Tc 99m.
After reconstitution, store refrigerated 2° to 8°C (36° to 46°F) • Use within 6 hours after reconstitution.
Contents:
10 sterile multi-dose reaction vials
20 radioactivity labels
Prescribing information
CardinalHealth™
NONRADIOACTIVE
Kit for the Preparation of Technetium Tc 99m Medronate
For preparation of technetium Tc 99m medronate for bone imaging
Each reaction vial contains a sterile, nonpyrogenic nonradioactive lyophilized
mixture of 20 mg medronic acid, 1 mg ascorbic acid, and 0.13 mg (minimum)
stannous fluoride SnF2 and 0.38 mg total tin, maximum (as stannous fluoride,
SnF2); pH adjusted with sodium hydroxide or hydrochloric acid prior to
lyophilization.
Distributed by
Cardinal Health 414, LLC
Dublin, OH 43017
Contains no preservative. Vial contents are
sealed under nitrogen at time of manufacture.
Recommended Dose: See Prescribing
Information
876659-H01
LOT 12345
YYY-MM-DD
INGREDIENTS AND APPEARANCE
| KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEDRONATE
technetium tc 99m medronate injection, powder, lyophilized, for solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Cardinal Health 414, LLC (069410546) |