Search by Drug Name or NDC
NDC 66885-0051-34 KELSAN Antimicrobial Ltion Skin Cleanser 0.003 mg/mL Details
KELSAN Antimicrobial Ltion Skin Cleanser 0.003 mg/mL
KELSAN Antimicrobial Ltion Skin Cleanser is a TOPICAL LIQUID in the HUMAN OTC DRUG category. It is labeled and distributed by Kel-San, Inc.. The primary component is CHLOROXYLENOL.
Product Information
| NDC | 66885-0051 |
|---|---|
| Product ID | 66885-051_35d13785-c0fe-4b00-99de-ffedd5b7b9c2 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | KELSAN Antimicrobial Ltion Skin Cleanser |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Chloroxylenol |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | LIQUID |
| Route | TOPICAL |
| Active Ingredient Strength | 0.003 |
| Active Ingredient Units | mg/mL |
| Substance Name | CHLOROXYLENOL |
| Labeler Name | Kel-San, Inc. |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part333E |
| Listing Certified Through | n/a |
Package
Package Images
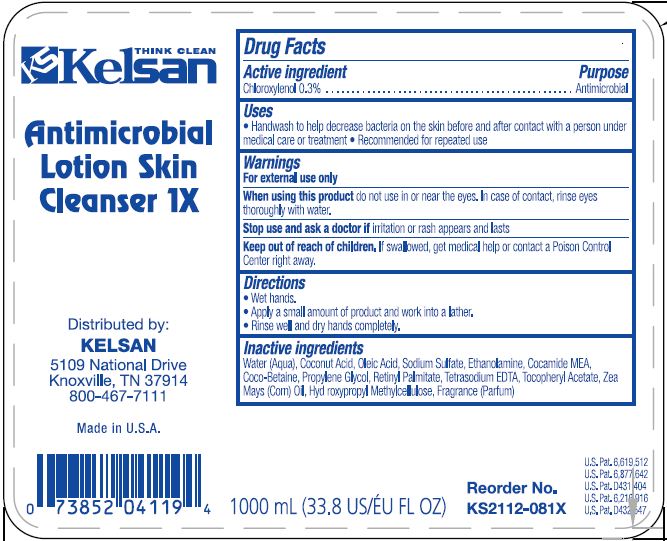
NDC 66885-0051-34 (66885005134)
| NDC Package Code | 66885-051-34 |
|---|---|
| Billing NDC | 66885005134 |
| Package | 1000 mL in 1 PACKAGE (66885-051-34) |
| Marketing Start Date | 2000-06-30 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 3cfeae6b-0b6a-4894-b8c0-85df830989cd Details
Warnings
Directions
Inactive ingredients
INGREDIENTS AND APPEARANCE
| KELSAN ANTIMICROBIAL LTION SKIN CLEANSER
chloroxylenol liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Kel-San, Inc. (108349937) |
Revised: 12/2020
Document Id: 35d13785-c0fe-4b00-99de-ffedd5b7b9c2
Set id: 3cfeae6b-0b6a-4894-b8c0-85df830989cd
Version: 2
Effective Time: 20201222