Search by Drug Name or NDC
NDC 67939-0010-50 Fludeoxyglucose F 18 300 mCi/mL Details
Fludeoxyglucose F 18 300 mCi/mL
Fludeoxyglucose F 18 is a INTRAVENOUS INJECTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by The University of Utah DBA Cyclotron Radiochemistry Lab / Huntsman Cancer Institute. The primary component is FLUDEOXYGLUCOSE F-18.
Product Information
| NDC | 67939-0010 |
|---|---|
| Product ID | 67939-010_d93db56f-430e-5490-e053-2995a90ace22 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Fludeoxyglucose F 18 |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Fludeoxyglucose F 18 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 300 |
| Active Ingredient Units | mCi/mL |
| Substance Name | FLUDEOXYGLUCOSE F-18 |
| Labeler Name | The University of Utah DBA Cyclotron Radiochemistry Lab / Huntsman Cancer Institute |
| Pharmaceutical Class | Radioactive Diagnostic Agent [EPC], Radiopharmaceutical Activity [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA204498 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

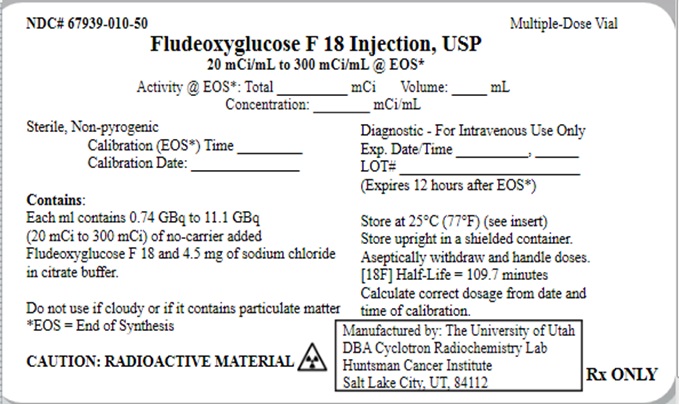


NDC 67939-0010-50 (67939001050)
| NDC Package Code | 67939-010-50 |
|---|---|
| Billing NDC | 67939001050 |
| Package | 50 mL in 1 VIAL, MULTI-DOSE (67939-010-50) |
| Marketing Start Date | 2020-05-20 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 4439286d-2b7b-4403-9e4a-3b0aad10ad5b Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
Fludeoxyglucose F18 Injection, USP
For intravenous use
Initial U.S. Approval: 2005
INDICATIONS AND USAGE
Fludeoxyglucose F18 Injection, USP is indicated for positron emission tomography (PET) imaging in the following settings: (1)
• Oncology: For assessment of abnormal glucose metabolism to assist in the evaluation of malignancy in patients with known or suspected abnormalities found by other testing modalities, or in patients with an existing diagnosis of cancer. (1)
• Cardiology: For the identification of left ventricular myocardium with residual glucose metabolism and reversible loss of systolic function in patients with coronary artery disease and left ventricular dysfunction, when used together with myocardial perfusion imaging. (1)
• Neurology: For the identification of regions of abnormal glucose metabolism associated with foci of epileptic seizures (1). (1)
DOSAGE AND ADMINISTRATION
Fludeoxyglucose F18 Injection emits radiation. Use procedures to minimize radiation exposure. Screen for blood glucose abnormalities. (2)
• In the oncology and neurology settings, instruct patients to fast for 4 – 6 hours prior to the drug’s injection. Consider medical therapy and laboratory testing to assure at least two days of normoglycemia prior to the drug’s administration (5.2). (2)
• In the cardiology setting, administration of glucose-containing food or liquids (e.g., 50 – 75 grams) prior to the drug’s injection facilitates localization of cardiac ischemia (2.3). (2)
Aseptically withdraw Fludeoxyglucose F18 Injection from its container and administer by intravenous injection (2). The recommended dose: (2)
• for adults is 5 – 10 mCi (185 – 370 MBq), in all indicated clinical settings (2.1). (2)
• for pediatric patients is 2.6 mCi in the neurology setting (2.2). Initiate imaging within 40 minutes following drug injection; acquire static emission images 30 – 100 minutes from time of injection (2). (2)
DOSAGE FORMS AND STRENGTHS
Multiple-dose glass vial containing 0.74 – 11.1 GBq (20 – 300 mCi/mL) of Fludeoxyglucose F18 Injection and 4.5 mg of sodium chloride in citrate buffer (approximately 35 mL or 50 mL volume), for intravenous administration (3). (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Hypersensitivity reactions have occurred; have emergency resuscitation equipment and personnel immediately available (6). (6)
(6)
To report SUSPECTED ADVERSE REACTIONS, contact The University of Utah DBA Cyclotron Radiochemistry Lab Huntsman Cancer Institute at 801-581-8745 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
USE IN SPECIFIC POPULATIONS
• Lactation: Temporarily discontinue breastfeeding. A lactating woman should pump and discard breastmilk for 9 hours after Fludeoxyglucose F 18 Injection (8.2). (8)
• Pediatric Use: Safety and effectiveness in pediatric patients have not been established in the oncology and cardiology settings (8.4). (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2020
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Oncology
1.2 Cardiology
1.3 Neurology
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose for Adults
2.2 Recommended Dose for Pediatric Patients
2.3 Patient Preparation
2.4 Radiation Dosimetry
2.5 Radiation Safety – Drug Handling
2.6 Drug Preparation and Administration
2.7 Imaging Guidelines
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Radiation Risks
5.2 Blood Glucose Abnormalities
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Oncology
14.2 Cardiology
14.3 Neurology
16 HOW SUPPLIED/STORAGE AND DRUG HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
Fludeoxyglucose F-18 Injection, USP is indicated for positron emission tomography (PET) imaging in the following settings:
1.1 Oncology
For assessment of abnormal glucose metabolism to assist in the evaluation of malignancy in patients with known or suspected abnormalities found by other testing modalities, or in patients with an existing diagnosis of cancer.
2 DOSAGE AND ADMINISTRATION
Fludeoxyglucose F-18 Injection emits radiation. Use procedures to minimize radiation exposure. Calculate the final dose from the end of synthesis (EOS) time using proper radioactive decay factors. Assay the final dose in a properly calibrated dose calibrator before administration to the patient [see Description(11.2)].
2.1 Recommended Dose for Adults
Within the oncology, cardiology and neurology settings, the recommended dose for adults is 5 – 10 mCi (185 – 370 MBq) as an intravenous injection.
2.2 Recommended Dose for Pediatric Patients
Within the neurology setting, the recommended dose for pediatric patients is 2.6 mCi, as an intravenous injection. The optimal dose adjustment on the basis of body size or weight has not been determined [see Use in Special Populations (8.4)].
2.3 Patient Preparation
- To minimize the radiation absorbed dose to the bladder, encourage adequate hydration. Encourage the patient to drink water or other fluids (as tolerated) in the 4 hours before their PET study.
-
Encourage the patient to void as soon as the imaging study is completed and as often as possible thereafter for at least one hour.
-
Screen patients for clinically significant blood glucose abnormalities by obtaining a history and/or laboratory tests [see Warnings and Precautions (5.2)]. Prior to Fludeoxyglucose F-18 PET imaging in the oncology and neurology settings, instruct patient to fast for 4 – 6 hours prior to the drug’s injection.
-
In the cardiology setting, administration of glucose-containing food or liquids (e.g., 50 – 75 grams) prior to Fludeoxyglucose F-18 Injection facilitates localization of cardiac ischemia.
2.4 Radiation Dosimetry
The estimated human absorbed radiation doses (rem/mCi) to a newborn (3.4 kg), 1-year old (9.8 kg), 5-year old (19 kg), 10-year old (32 kg), 15-year old (57 kg), and adult (70 kg) from intravenous administration of Fludeoxyglucose F-18 Injection are shown in Table 1. These estimates were calculated based on human 1 data and using the data published by the International Commission on Radiological Protection 2 for Fludeoxyglucose 18F. The dosimetry data show that there are slight variations in absorbed radiation dose for various organs in each of the age groups. These dissimilarities in absorbed radiation dose are due to developmental age variations (e.g., organ size, location, and overall metabolic rate for each age group). The identified critical organs (in descending order) across all age groups evaluated are the urinary bladder, heart, pancreas, spleen, and lungs.
| Organ | Newborn (3.4 kg) | 1-year old (9.8 kg) | 5-year old (19 kg) | 10-year old (32 kg) | 15-year old (57 kg) | Adult
(70 kg) |
| Bladder wall† | 4.3 | 1.7 | 0.93 | 0.60 | 0.40 | 0.32 |
| Heart wall | 2.4 | 1.2 | 0.70 | 0.44 | 0.29 | 0.22 |
| Pancreas | 2.2 | 0.68 | 0.33 | 0.25 | 0.13 | 0.096 |
| Spleen | 2.2 | 0.84 | 0.46 | 0.29 | 0.19 | 0.14 |
| Lungs | 0.96 | 0.38 | 0.20 | 0.13 | 0.092 | 0.064 |
| Kidneys | 0.81 | 0.34 | 0.19 | 0.13 | 0.089 | 0.074 |
| Ovaries | 0.80 | 0.8 | 0.19 | 0.11 | 0.058 | 0.053 |
| Uterus | 0.79 | 0.35 | 0.19 | 0.12 | 0.076 | 0.062 |
| LLI wall‡ | 0.69 | 0.28 | 0.15 | 0.097 | 0.060 | 0.051 |
| Liver | 0.69 | 0.31 | 0.17 | 0.11 | 0.076 | 0.058 |
| Gallbladder wall | 0.69 | 0.26 | 0.14 | 0.093 | 0.059 | 0.049 |
| Small intestine | 0.68 | 0.29 | 0.15 | 0.096 | 0.060 | 0.047 |
| ULI wall₴ | 0.67 | 0.27 | 0.15 | 0.090 | 0.057 | 0.046 |
| Stomach wall | 0.65 | 0.27 | 0.14 | 0.089 | 0.057 | 0.047 |
| Adrenals | 0.65 | 0.28 | 0.15 | 0.095 | 0.061 | 0.048 |
| Testes | 0.64 | 0.27 | 0.14 | 0.085 | 0.052 | 0.041 |
| Red marrow | 0.62 | 0.26 | 0.14 | 0.089 | 0.057 | 0.047 |
| Thymus | 0.61 | 0.26 | 0.14 | 0.086 | 0.056 | 0.044 |
| Thyroid | 0.61 | 0.26 | 0.13 | 0.080 | 0.049 | 0.039 |
| Muscle | 0.58 | 0.25 | 0.13 | 0.078 | 0.049 | 0.039 |
| Bone surface | 0.57 | 0.24 | 0.12 | 0.079 | 0.052 | 0.041 |
| Breast | 0.54 | 0.22 | 0.11 | 0.068 | 0.043 | 0.034 |
| Skin | 0.49 | 0.20 | 0.10 | 0.060 | 0.037 | 0.030 |
| Brain | 0.29 | 0.13 | 0.09 | 0.078 | 0.072 | 0.070 |
| Other tissues | 0.59 | 0.25 | 0.13 | 0.083 | 0.052 | 0.042 |
* MIRDOSE 2 software was used to calculate the radiation absorbed dose. Assumptions on the biodistribution based on data from Gallagher et al. 1 and Jones et al. 2
† The dynamic bladder model with a uniform voiding frequency of 1.5 hours was used.
‡ LLI = lower large intestine;
₴ ULI = upper large intestine
2.5 Radiation Safety – Drug Handling
- Use waterproof gloves, effective radiation shielding, and appropriate safety measures when handling Fludeoxyglucose F-18 Injection to avoid unnecessary radiation exposure to the patient, occupational workers, clinical personnel and other persons.
- Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
- Calculate the final dose from the end of synthesis (EOS) time using proper radioactive decay factors. Assay the final dose in a properly calibrated dose calibrator before administration to the patient [see Description (11.2)].
- The dose of Fludeoxyglucose F-18 used in a given patient should be minimized consistent with the objectives of the procedure, and the nature of the radiation detection devices employed.
2.6 Drug Preparation and Administration
- Calculate the necessary volume to administer based on calibration time and dose.
- Aseptically withdraw Fludeoxyglucose F-18 Injection from its container.
- Inspect Fludeoxyglucose F-18 Injection visually for particulate matter and discoloration before administration, whenever solution and container permit.
- Do not administer the drug if it contains particulate matter or discoloration; dispose of these unacceptable or unused preparations in a safe manner, in compliance with applicable regulations.
- Use Fludeoxyglucose F-18 Injection within 12 hours from the EOS.
3 DOSAGE FORMS AND STRENGTHS
5 WARNINGS AND PRECAUTIONS
5.1 Radiation Risks
Radiation-emitting products, including Fludeoxyglucose F-18 Injection, may increase the risk for cancer, especially in pediatric patients. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker [see Dosage and Administration(2.5)].
5.2 Blood Glucose Abnormalities
In the oncology and neurology setting, suboptimal imaging may occur in patients with inadequately regulated blood glucose levels. In these patients, consider medical therapy and laboratory testing to assure at least two days of normoglycemia prior to Fludeoxyglucose F-18 Injection administration.
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Data from published case series and case reports describe Fludeoxyglucose F 18 Injection crossing the placenta with uptake by the fetus (see Data). All radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. However, published studies that describe Fludeoxyglucose F 18 Injection use in pregnant women have not identified a risk of drug-associated major birth defects, miscarriage, or adverse maternal or fetal outcomes. If considering Fludeoxyglucose F 18 Injection administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from Fludeoxyglucose F 18 Injection and the gestational timing of exposure.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Data
Human Data
Data from published case series and case reports describe Fludeoxyglucose F 18 Injection crossing the placental barrier and visualization of radioactivity throughout the body of the fetus. The estimated fetal absorbed radiation dose from the maximum labeled dose (370 MBq) of Fludeoxyglucose F 18 was 10mGy with first trimester exposure to PET alone and 20mGy with first trimester exposure to PET/CT scan combination. Long-term adverse radiation effects to a child exposed to Fludeoxyglucose F 18 Injection in utero are unknown. No adverse fetal effects or radiation-related risks have been identified for diagnostic procedures involving less than 50mGy, which represents less than 20mGy fetal doses.
8.2 Lactation
Risk Summary
A published case report and case series show the presence of Fludeoxyglucose F 18 Injection in human milk following administration. There are no data on the effects of Fludeoxyglucose F 18 Injection on the breastfed infant or the effects on milk production. Exposure of Fludeoxyglucose F 18 Injection to a breastfed infant can be minimized by temporary discontinuation of breastfeeding (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Fludeoxyglucose F 18 Injection, any potential adverse effects on the breastfed child from Fludeoxyglucose F 18 Injection or from the underlying maternal condition.
Clinical Considerations
To decrease radiation exposure to the breastfed infant, advise a lactating woman to pump and discard breastmilk and avoid close (breast) contact with the infant for at least 9 hours after the administration of Fludeoxyglucose F 18 Injection.
8.4 Pediatric Use
The safety and effectiveness of Fludeoxyglucose F-18 Injection in pediatric patients with epilepsy is established on the basis of studies in adult and pediatric patients. In pediatric patients with epilepsy, the recommended dose is 2.6 mCi. The optimal dose adjustment on the basis of body size or weight has not been determined. In the oncology or cardiology settings, the safety and effectiveness of Fludeoxyglucose F-18 Injection have not been established in pediatric patients.
11 DESCRIPTION
11.1 Chemical Characteristics
Fludeoxyglucose F-18 Injection, USP is a positron emitting radiopharmaceutical that is used for diagnostic purposes in conjunction with positron emission tomography (PET) imaging. The active ingredient 2-deoxy-2-[ 18F]fluoro-D-glucose has the molecular formula of C 6H 1118 FO 5 with a molecular weight of 181.26, and has the following chemical structure:

Fludeoxyglucose F-18 Injection, USP is provided as a ready to use sterile, pyrogen free, clear, colorless citrate buffered solution. Each mL contains between 0.740 to 11.1GBq (20.0-300 mCi) of 2-deoxy-2- [ 18F]fluoro-D-glucose at the EOS, 4.5 mg of sodium chloride in citrate buffer. The pH of the solution is between 4.5 and 7.5. The solution is packaged in a multiple-dose glass vial and does not contain any preservative.
11.2 Physical Characteristics
Fluorine F-18 decays by emitting positron to Oxygen O 18 (stable) and has a physical half-life of 109.7 minutes. The principal photons useful for imaging are the dual 511 keV gamma photons, that are produced and emitted simultaneously in opposite direction when the positron interacts with an electron (Table 2).
| Radiation/Emission | % Per Disintegration | Mean Energy |
| Positron(β+) | 96.73 | 249.8 keV |
| Gamma(±)* | 193.46 | 511.0 keV |
*Produced by positron annihilation
From: Kocher, D.C. Radioactive Decay Tables DOE/TIC-I 1026, 89 (1981)
The specific gamma ray constant (point source air kerma coefficient) for fluorine F-18 is 5.7 R/hr/mCi (1.35 x 10
-6 Gy/hr/kBq) at 1 cm. The half-value layer (HVL) for the 511 keV photons is 4 mm lead (Pb). The range of attenuation coefficients for this radionuclide as a function of lead shield thickness is shown in Table 3. For example, the interposition of an 8 mm thickness of Pb, with a coefficient of attenuation of 0.25, will decrease the external radiation by 75%.
| Shield thickness (Pb) mm | Coefficient of attenuation |
| 0 | 0.00 |
| 4 | 0.50 |
| 8 | 0.25 |
| 13 | 0.10 |
| 26 | 0.01 |
| 39 | 0.001 |
| 52 | 0.0001 |
For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 4.
| Minutes | Fraction Remaining |
| 0* | 1.000 |
| 15 | 0.909 |
| 30 | 0.826 |
| 60 | 0.683 |
| 110 | 0.500 |
| 220 | 0.250 |
*calibration time
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fludeoxyglucose F-18 is a glucose analog that concentrates in cells that rely upon glucose as an energy source, or in cells whose dependence on glucose increases under pathophysiological conditions. Fludeoxyglucose F-18 is transported through the cell membrane by facilitative glucose transporter proteins and is phosphorylated within the cell to [ 18F] FDG-6-phosphate bythe enzyme hexokinase. Once phosphorylated it cannot exit until it is dephosphorylated by glucose-6-phosphatase. Therefore, within a given tissue or pathophysiological process, the retention and clearance of Fludeoxyglucose F-18 reflect a balance involving glucose transporter, hexokinase and glucose-6-phosphatase activities. Fludeoxyglucose F-18 is used to assess glucose metabolism.
In comparison to background activity of the specific organ or tissue type, regions of decreased or absent uptake of Fludeoxyglucose F 18 reflect the decrease or absence of glucose metabolism. Regions of increased uptake of Fludeoxyglucose F 18 reflect greater than normal rates of glucose metabolism.
12.2 Pharmacodynamics
Fludeoxyglucose F-18 Injection is rapidly distributed to all organs of the body after intravenous administration. After background clearance of Fludeoxyglucose F-18 Injection, optimal PET imaging is generally achieved between 30 to 40 minutes after administration.
In cancer, the cells are generally characterized by enhanced glucose metabolism partially due to (1) an increase in activity of glucose transporters, (2) an increased rate of phosphorylation activity, (3) a reduction of phosphatase activity or, (4) a dynamic alteration in the balance among all these processes. However, glucose metabolism of cancer as reflected by Fludeoxyglucose F-18 accumulation shows considerable variability. Depending on tumor type, stage, and location, Fludeoxyglucose F-18 accumulation may be increased, normal, or decreased. Also, inflammatory cells can have the same variability of uptake of Fludeoxyglucose F-18.
In the heart, under normal aerobic conditions, the myocardium meets the bulk of its energy requirements by oxidizing free fatty acids. Most of the exogenous glucose taken up by the myocyte is converted into glycogen. However, under ischemic conditions, the oxidation of free fatty acids decreases, exogenous glucose becomes the preferred myocardial substrate, glycolysis is stimulated, and glucose taken up by the myocyte is metabolized immediately instead of being converted into glycogen. Under these conditions, phosphorylated Fludeoxyglucose F-18 accumulates in the myocyte and can be detected with PET imaging.
In the brain, cells normally rely on aerobic metabolism. In epilepsy, the glucose metabolism varies. Generally, during a seizure, glucose metabolism increases. Interictally, the seizure focus tends to be hypometabolic.
12.3 Pharmacokinetics
Distribution:In four healthy male volunteers, receiving an intravenous administration of 30 seconds in duration, the arterial blood level profile for Fludeoxyglucose F-18 decayed triexponentially. The effective half-life ranges of the three phases were 0.2-0.3 minutes, 10-13 minutes with a mean and standard deviation (STD) of 11.6 (±) 1.1 min, and 80-95 minutes with a mean and STD of 88 (±) 4 min.
Plasma protein binding of Fludeoxyglucose F-18 has not been studied.
Metabolism: Fludeoxyglucose F-18 is transported into cells and phosphorylated to [ 18F]-FDG-6-phosphate at a rate proportional to the rate of glucose utilization within that tissue. [18F]-FDG-6-phosphate presumably is metabolized to 2-deoxy-2-[ 18F]fluoro-6-phospho-Dmannose([ 18F]FDM-6-phosphate).
Fludeoxyglucose F-18 Injection may contain several impurities (e.g., 2-deoxy-2-chloro-Dglucose (ClDG)). Biodistribution and metabolism of ClDG are presumed to be similar to Fludeoxyglucose F-18 and would be expected to result in intracellular formation of 2-deoxy-2-chloro-6-phospho-D-glucose (ClDG-6-phosphate) and 2-deoxy-2-chloro-6-phospho-D-mannose (ClDM-6-phosphate). The phosphorylated deoxyglucose compounds are dephosphorylated and the resulting compounds (FDG, FDM, ClDG, and ClDM) presumably leave cells by passive diffusion. Fludeoxyglucose F-18 and related compounds are cleared from non-cardiac tissues within 3 to 24 hours after administration. Clearance from the cardiac tissue may require more than 96 hours. Fludeoxyglucose F-18 that is not involved in glucose metabolism in any tissue is then excreted in the urine.
Elimination:Fludeoxyglucose F-18 is cleared from most tissues within 24 hours and can be eliminated from the body unchanged in the urine. Three elimination phases have been identified in the reviewed literature. Within 33 minutes, a mean of 3.9% of the administrated radioactive dose was measured in the urine. The amount of radiation exposure of the urinary bladder at two hours post-administration suggests that 20.6% (mean) of the radioactive dose was present in the bladder.
Special Populations:
The pharmacokinetics of Fludeoxyglucose F-18 Injection have not been studied in renally-impaired, hepatically impaired or pediatric patients. Fludeoxyglucose F-18 is eliminated through the renal system. Avoid excessive radiation exposure to this organ system and adjacent tissues.
The effects of fasting, varying blood sugar levels, conditions of glucose intolerance, and diabetes mellitus on Fludeoxyglucose F-18 distribution in humans have not been ascertained [ see Warnings and Precautions (5.2)].
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
14.1 Oncology
The efficacy of Fludeoxyglucose F-18 Injection in positron emission tomography cancer imaging was demonstrated in 16 independent studies. These studies prospectively evaluated the use of Fludeoxyglucose F-18 in patients with suspected or known malignancies, including non-small cell lung cancer, colo-rectal, pancreatic, breast, thyroid, melanoma, Hodgkin's and non-Hodgkin's lymphoma, and various types of metastatic cancers to lung, liver, bone, and axillary nodes. All these studies had at least 50 patients and used pathology as a standard of truth. The Fludeoxyglucose F-18 Injection doses in the studies ranged from 200 MBq to 740 MBq with a median and mean dose of 370 MBq.
In the studies, the diagnostic performance of Fludeoxyglucose F-18 Injection varied with the type of cancer, size of cancer, and other clinical conditions. False negative and false positive scans were observed. Negative Fludeoxyglucose F-18 Injection PET scans do not exclude the diagnosis of cancer. Positive Fludeoxyglucose F-18 Injection PET scans can not replace pathology to establish a diagnosis of cancer. Non-malignant conditions such as fungal infections, inflammatory processes and benign tumors have patterns of increased glucose metabolism that may give rise to false-positive scans. The efficacy of Fludeoxyglucose F-18 Injection PET imaging in cancer screening was not studied.
14.2 Cardiology
The efficacy of Fludeoxyglucose F-18 Injection for cardiac use was demonstrated in ten independent, prospective studies of patients with coronary artery disease and chronic left ventricular systolic dysfunction who were scheduled to undergo coronary revascularization. Before revascularization, patients underwent PET imaging with Fludeoxyglucose F-18 Injection (74 – 370 MBq, 2 – 10 mCi) and perfusion imaging with other diagnostic radiopharmaceuticals. Doses of Fludeoxyglucose F-18 Injection ranged from 74-370 MBq (2-10 mCi). Segmental, left ventricular, wall-motion assessments of asynergic areas made before revascularization were compared in a blinded manner to assessments made after successful revascularization to identify myocardial segments with functional recovery.
Left ventricular myocardial segments were predicted to have reversible loss of systolic function if they showed Fludeoxyglucose F-18 accumulation and reduced perfusion (i.e., flow-metabolism mismatch). Conversely, myocardial segments were predicted to have irreversible loss of systolic function if they showed reductions in both Fludeoxyglucose F- 18 accumulation and perfusion (i.e., matched defects).
Findings of flow-metabolism mismatch in a myocardial segment may suggest that successful revascularization will restore myocardial function in that segment. However, false-positive tests occur regularly, and the decision to have a patient undergo revascularization should not be based on PET findings alone. Similarly, findings of a matched defect in a myocardial segment may suggest that myocardial function will not recover in that segment, even if it is successfully revascularized. However, false-negative tests occur regularly, and the decision to recommend against coronary revascularization, or to recommend a cardiac transplant, should not be based on PET findings alone. The reversibility of segmental dysfunction as predicted with Fludeoxyglucose F-18 PET imaging depends on successful coronary revascularization. Therefore, in patients with a low likelihood of successful revascularization, the diagnostic usefulness of PET imaging with Fludeoxyglucose F-18 Injection is more limited.
14.3 Neurology
In a prospective, open label trial, Fludeoxyglucose F-18 Injection was evaluated in 86 patients with epilepsy. Each patient received a dose of Fludeoxyglucose F-18 Injection in the range of 185-370 MBq (5-10 mCi). The mean age was 16.4 years (range: 4 months - 58 years; of these, 42 patients were less than 12 years and 16 patients were less than 2 years old). Patients had a known diagnosis of complex partial epilepsy and were under evaluation for surgical treatment of their seizure disorder. Seizure foci had been previously identified on ictal EEGs and sphenoidal EEGs. Fludeoxyglucose F-18 Injection PET imaging confirmed previous diagnostic findings in 16% (14/87) of the patients; in 34% (30/87) of the patients, Fludeoxyglucose F-18 Injection PET images provided new findings. In 32% (27/87), imaging with Fludeoxyglucose F-18 Injection was inconclusive. The impact of these imaging findings on clinical outcomes is not known.
Several other studies comparing imaging with Fludeoxyglucose F-18 Injection results to subsphenoidal EEG, MRI and/or surgical findings supported the concept that the degree of hypometabolism corresponds to areas of confirmed epileptogenic foci. The safety and effectiveness of Fludeoxyglucose F-18 Injection to distinguish idiopathic epileptogenic foci from tumors or other brain lesions that may cause seizures have not been established.
16 HOW SUPPLIED/STORAGE AND DRUG HANDLING
Fludeoxyglucose F-18 Injection, USP is supplied in a multi-dose, capped 50 mL glass vial containing between 0.740 – 11.1 GBq/mL (20 - 300 mCi/mL), of no carrier added 2-deoxy-2-[18F] fluoro-D-glucose, at end of synthesis, in approximately 35 mL or 50 mL. The contents of each vial are sterile, pyrogen-free and preservative-free.
NDC 67939-010-50
Store the Fludeoxyglucose F-18 Injection, USP vial upright in a lead shielded container at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Store and dispose of Fludeoxyglucose F-18 Injection, USP in accordance with the regulations and a general license, or its equivalent, of an Agreement State or a Licensing State.
The expiration date and time are provided on the container label. Use Fludeoxyglucose F18
Injection, USP within 12 hours from the EOS time.
17 PATIENT COUNSELING INFORMATION
Instruct patients in procedures that increase renal clearance of radioactivity. Encourage patients to:
- drink water or other fluids (as tolerated) in the 4 hours before their PET study.
- void as soon as the imaging study is completed and as often as possible thereafter for at least one hour.
Pregnancy: Advise pregnant women of the risk of fetal exposure to radiation with Fludeoxyglucose F 18 Injection [
see Use in Specific Populations (8.1)].
Lactation: Advise lactating women that exposure to Fludeoxyglucose F 18 Injection through breast milk can be minimized by pumping and discarding breast milk and avoiding close (breast) contact with the infant for 9 hours after Fludeoxyglucose F 18 Injection
[see Use in Specific Populations (8.2)].
| Manufactured by: | The University of Utah DBA Cyclotron Radiochemistry Lab Hunstman Cancer Institute |
| 2000 Circle of Hope | |
| Salt Lake City, UT 84112 | |
| Distributed by: | The University of Utah DBA Cyclotron Radiochemistry Lab Hunstman Cancer Institute |
| 2000 Circle of Hope | |
| Salt Lake City, UT 84112 |
Outer Package Labels & Vial Labels
INGREDIENTS AND APPEARANCE
| FLUDEOXYGLUCOSE F 18
fludeoxyglucose f 18 injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - The University of Utah DBA Cyclotron Radiochemistry Lab / Huntsman Cancer Institute (018432646) |
| Registrant - The University of Utah DBA Cyclotron Radiochemistry Lab / Huntsman Cancer Institute (018432646) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The University of Utah DBA Cyclotron Radiochemistry Lab / Huntsman Cancer Institute | 018432646 | positron emission tomography drug production(67939-010) | |