Search by Drug Name or NDC
NDC 70860-0403-51 Bivalirudin 5 mg/mL Details
Bivalirudin 5 mg/mL
Bivalirudin is a INTRAVENOUS INJECTION, SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Athenex Pharmaceutical Division, LLC.. The primary component is BIVALIRUDIN.
Product Information
| NDC | 70860-0403 |
|---|---|
| Product ID | 70860-403_e670c25e-1572-4492-a6b5-4f42ba4b389a |
| Associated GPIs | 83334020202020 |
| GCN Sequence Number | 080076 |
| GCN Sequence Number Description | bivalirudin VIAL 250MG/50ML INTRAVEN |
| HIC3 | M9E |
| HIC3 Description | THROMBIN INHIBITORS,SEL,DIRECT,REVERS-HIRUDIN TYPE |
| GCN | 46763 |
| HICL Sequence Number | 021872 |
| HICL Sequence Number Description | BIVALIRUDIN |
| Brand/Generic | Generic |
| Proprietary Name | Bivalirudin |
| Proprietary Name Suffix | RTU |
| Non-Proprietary Name | bivalirudin |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, SOLUTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 5 |
| Active Ingredient Units | mg/mL |
| Substance Name | BIVALIRUDIN |
| Labeler Name | Athenex Pharmaceutical Division, LLC. |
| Pharmaceutical Class | Anti-coagulant [EPC], Direct Thrombin Inhibitor [EPC], Thrombin Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA211215 |
| Listing Certified Through | 2022-12-31 |
Package
Package Images

NDC 70860-0403-51 (70860040351)
| NDC Package Code | 70860-403-51 |
|---|---|
| Billing NDC | 70860040351 |
| Package | 10 CARTON in 1 CARTON (70860-403-51) / 1 VIAL, SINGLE-DOSE in 1 CARTON (70860-403-50) / 50 mL in 1 VIAL, SINGLE-DOSE |
| Marketing Start Date | 2020-03-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 1ed8c822-6fe1-49c1-a59f-31a4c72818c8 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
BIVALIRUDIN RTU (bivalirudin) injection, for intravenous use
Initial U.S. Approval: 2000
INDICATIONS AND USAGE
Bivalirudin RTU Injection is a direct thrombin inhibitor indicated for use as an anticoagulant in patients undergoing percutaneous coronary intervention (PCI), including patients with heparin-induced thrombocytopenia and heparin-induced thrombocytopenia and thrombosis syndrome. (1)
DOSAGE AND ADMINISTRATION
- The recommended dosage is a 0.75 mg/kg intravenous bolus dose followed immediately by a 1.75 mg/kg/h intravenous infusion for the duration of the procedure. Five minutes after the bolus dose, assess activated clotting time (ACT) to determine if an additional bolus of 0.3 mg/kg is needed. (2.1)
- Consider extending duration of infusion post-procedure up to 4 hours in patients with ST segment elevation MI. (2.1)
DOSAGE FORMS AND STRENGTHS
Injection: 250 mg per 50 mL (5 mg per mL) in a single-dose vial. Ready-to-use. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Bleeding events: bivalirudin increases the risk of bleeding. Its anticoagulant effect subsides approximately one hour after discontinuation. (5.1, 6.1, 12.2)
- Thrombotic risk with coronary artery brachytherapy: An increased risk of thrombus formation, including fatal outcomes, in gamma brachytherapy. (5.2, 6.2)
ADVERSE REACTIONS
Most common adverse reaction was bleeding (3.7%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Athenex Pharmaceutical Division, LLC. at 1-855-273-0154 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Heparin, warfarin, thrombolytics, or GPIs: Increased major bleeding risk with concomitant use. (7)
USE IN SPECIFIC POPULATIONS
Geriatric patients: Increased bleeding risk possible. (8.5) Renal impairment: Reduce infusion dose and monitor ACT. (2.2, 8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dose Adjustment in Renal Impairment
2.3 Instructions for Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bleeding Events
5.2 Thrombotic Risk with Coronary Artery Brachytherapy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of Bivalirudin RTU Injection is an intravenous bolus dose of 0.75 mg/kg, followed immediately by a maintenance infusion of 1.75 mg/kg/h for the duration of the procedure. Five minutes after the bolus dose has been administered, assess activated clotting time (ACT) to determine if an additional bolus of 0.3 mg/kg is needed.
Consider extending duration of infusion following PCI at 1.75 mg/kg/h for up to 4 hours post-procedure in patients with ST segment elevation MI (STEMI).
2.3 Instructions for Administration
Inspection of Container
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Once removed from refrigerator, use immediately [see How Supplied Storage and Handling (16.2)]. Discard any unused portion.
Drug Compatibilities
No incompatibilities have been observed with administration sets.
Do not administer the drugs listed in Table 1 in the same intravenous line with Bivalirudin RTU Injection.
| Alteplase |
| Amiodarone HCl |
| Amphotericin B |
| Chlorpromazine HCl |
| Diazepam |
| Dobutamine |
| Prochlorperazine Edisylate |
| Reteplase |
| Streptokinase |
| Vancomycin HCl |
3 DOSAGE FORMS AND STRENGTHS
Bivalirudin RTU Injection, clear to slightly opalescent, colorless to yellow sterile solution:
- 250 mg of bivalirudin per 50 mL (5 mg per mL) in a single-dose vial. Ready-to-use. Each vial contains 250 mg of bivalirudin equivalent to an average of 275 mg bivalirudin trifluoroacetate*.
*The range of bivalirudin trifluoroacetate is 270 to 280 mg based on a range of trifluoroacetic acid composition of 1.7 to 2.6 equivalents.
4 CONTRAINDICATIONS
Bivalirudin RTU Injection is contraindicated in patients with:
- Significant active bleeding;
- Hypersensitivity to Bivalirudin RTU Injection or its components [see Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Bleeding Events
Bivalirudin increases the risk of bleeding [see Adverse Reactions (6.1)]. Bivalirudin's anticoagulant effect subsides approximately one hour after discontinuation [see Clinical Pharmacology (12.2)].
5.2 Thrombotic Risk with Coronary Artery Brachytherapy
An increased risk of thrombus formation, including fatal outcomes, has been associated with the use of bivalirudin in gamma brachytherapy [see Adverse Reactions (6.2)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the BAT trials, 79 of the 2161 (3.7%) of subjects undergoing PCI for treatment of unstable angina and randomized to bivalirudin experienced intracranial bleeding, retroperitoneal bleeding, clinically overt bleeding with a decrease in hemoglobin greater than 3 g/dL or leading to a transfusion of greater than 2 units of blood.
Immunogenicity/Re-Exposure
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to bivalirudin in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
In in vitro studies, bivalirudin exhibited no platelet aggregation response against sera from patients with a history of HIT/HITTS.
Among 494 subjects who received bivalirudin in clinical trials and were tested for antibodies, 2 subjects had treatment-emergent positive bivalirudin antibody tests. Neither subject demonstrated clinical evidence of allergic or anaphylactic reactions and repeat testing was not performed. Nine additional patients who had initial positive tests were negative on repeat testing.
6.2 Postmarketing Experience
Because postmarketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been identified during post-approval use of bivalirudin: fatal bleeding; hypersensitivity and allergic reactions including reports of anaphylaxis; lack of anticoagulant effect; thrombus formation during PCI with and without intracoronary brachytherapy, including reports of fatal outcomes; pulmonary hemorrhage; cardiac tamponade; and INR increased.
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on use of bivalirudin in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Reproduction studies in rats and rabbits administered subcutaneously (SC) doses up to 1.6 times and 3.2 times the maximum recommended human dose (MRHD) based on body surface area (BSA), respectively, revealed no evidence of fetal harm.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Reproductive studies have been performed in rats at subcutaneous doses up to 150 mg/kg/day, (1.6 times the maximum recommended human dose based on body surface area) and rabbits at subcutaneous doses up to 150 mg/kg/day (3.2 times the maximum recommended human dose based on body surface area). These studies revealed no harm to the fetus attributable to bivalirudin.
At 500 mg/kg/day subcutaneously, litter sizes and live fetuses in rats were reduced. Fetal skeletal variations were also noted. Some of these changes could be attributed to maternal toxicity observed at high doses.
8.4 Pediatric Use
The safety and effectiveness of bivalirudin in pediatric patients have not been established.
8.5 Geriatric Use
In studies of patients undergoing PCI, 44% were ≥65 years of age and 12% of patients were ≥75 years old. Elderly patients experienced more bleeding events than younger patients.
8.6 Renal Impairment
The disposition of bivalirudin was studied in PTCA patients with mild, moderate and severe renal impairment. The clearance of bivalirudin was reduced approximately 21% in patients with moderate and severe renal impairment and was reduced approximately 70% in dialysis-dependent patients [see Clinical Pharmacology (12.3)]. The infusion dose of Bivalirudin RTU Injection may need to be reduced, and anticoagulant status monitored in patients with renal impairment [see Dosage and Administration (2.2)].
10 OVERDOSAGE
Cases of overdose of up to 10 times the recommended bolus or continuous infusion dose of bivalirudin have been reported in clinical trials and in postmarketing reports. A number of the reported overdoses were due to failure to adjust the infusion dose of bivalirudin in persons with renal dysfunction including persons on hemodialysis [see Dosage and Administration (2.2)]. Bleeding, as well as deaths due to hemorrhage, have been observed in some reports of overdose. In cases of suspected overdosage, discontinue bivalirudin immediately and monitor the patient closely for signs of bleeding. There is no known antidote to bivalirudin. Bivalirudin is hemodialyzable [see Clinical Pharmacology (12.3)].
11 DESCRIPTION
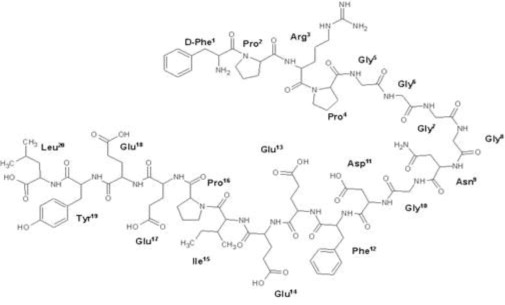
Bivalirudin RTU Injection contains bivalirudin trifluoroacetate, which is a specific and reversible direct thrombin inhibitor. Bivalirudin trifluoroacetate is a synthetic, 20 amino acid peptide salt, with the chemical name of D-phenylalanyl-L-prolyl-L-arginyl-L-prolylglycylglycylglycylglycyl-L-asparagylglycyl-L-α-aspartyl-L-phenylalanyl-L-α-glutamyl-L-α-glutamyl-L-isoleucyl-L-prolyl-L-α-glutamyl-L-α-glutamyl-L-tyrosyl-L-leucine trifluoroacetate. Each molecule of bivalirudin trifluoroacetate contains 1.7 to 2.6 equivalents of trifluoroacetic acid. The molecular formula of bivalirudin free base is C98H138N24O33 and its molecular weight is 2180.32 Daltons (anhydrous free base peptide). The structural formula of bivalirudin free base is:
Figure 1: Structural Formula of Bivalirudin
Bivalirudin RTU Injection is supplied as a refrigerated, ready-to-use, sterile solution packaged in a 50 mL single-dose vial. Each milliliter of Bivalirudin RTU Injection contains 5 mg bivalirudin (as trifluoroacetate salt)*, 0.8 mg sodium acetate trihydrate, 100 mg polyethylene glycol 400, and Water for Injection.
The pH of Bivalirudin RTU Injection may have been adjusted with sodium hydroxide and/or glacial acetic acid to 5.0 to 5.5. The solution is intended for intravenous administration at room temperature (20ºC to 25°C/68ºF to 77°F).
*The range of bivalirudin trifluoroacetate is 5.4 to 5.6 mg based on a range of trifluoroacetic acid composition of 1.7 to 2.6 equivalents.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bivalirudin directly inhibits thrombin by specifically binding both to the catalytic site and to the anion-binding exosite of circulating and clot-bound thrombin. Thrombin is a serine proteinase that plays a central role in the thrombotic process, acting to cleave fibrinogen into fibrin monomers and to activate Factor XIII to Factor XIIIa, allowing fibrin to develop a covalently cross-linked framework which stabilizes the thrombus; thrombin also activates Factors V and VIII, promoting further thrombin generation, and activates platelets, stimulating aggregation and granule release. The binding of bivalirudin to thrombin is reversible as thrombin slowly cleaves the bivalirudin-Arg3-Pro4 bond, resulting in recovery of thrombin active site functions.
12.2 Pharmacodynamics
In healthy volunteers and patients (with ≥70% vessel occlusion undergoing routine PTCA), bivalirudin exhibited dose- and concentration-dependent anticoagulant activity as evidenced by prolongation of the ACT, aPTT, PT, and TT. Intravenous administration of bivalirudin produces an immediate anticoagulant effect. Coagulation times return to baseline approximately 1 hour following cessation of bivalirudin administration. Bivalirudin also increases INR.
In 291 patients with ≥70% vessel occlusion undergoing routine PTCA, a positive correlation was observed between the dose of bivalirudin and the proportion of patients achieving ACT values of 300 sec or 350 sec. At a bivalirudin dose of 1 mg/kg intravenous bolus plus 2.5 mg/kg/h intravenous infusion (1.4 times higher than the approved dose of 1.75 mg/kg/h) for 4 hours, followed by 0.2 mg/kg/h, all patients reached maximal ACT values greater than 300 sec.
12.3 Pharmacokinetics
Bivalirudin exhibits linear pharmacokinetics following intravenous administration to patients undergoing PTCA. In these patients, a mean steady state bivalirudin concentration of 12.3 ± 1.7 mcg/mL is achieved following an intravenous bolus of 1 mg/kg and a 4-hour 2.5 mg/kg/h intravenous infusion.
Elimination
Bivalirudin has a half-life of 25 minutes in PTCA patients with normal renal function. The total body clearance of bivalirudin in PTCA patients with normal renal function is 3.4 mL/min/kg.
Specific Populations
Patients with Renal Impairment
Total body clearance was similar for PTCA patients with normal renal function and with mild renal impairment. Clearance was reduced by 21% in patients with moderate and severe renal impairment with a half-life of 34 and 57 minutes, respectively. In dialysis patients, clearance was reduced by 70%, with a half-life of 3.5 hours. Approximately 25% bivalirudin is cleared by hemodialysis.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of bivalirudin. Bivalirudin displayed no genotoxic potential in the in vitro bacterial cell reverse mutation assay (Ames test), the in vitro Chinese hamster ovary cell forward gene mutation test (CHO/HGPRT), the in vitro human lymphocyte chromosomal aberration assay, the in vitro rat hepatocyte unscheduled DNA synthesis (UDS) assay, and the in vitro rat micronucleus assay. Fertility and general reproductive performance in rats were unaffected by subcutaneous doses of bivalirudin up to 150 mg/kg/day, about 1.6 times the dose on a body surface area basis (mg/m2) of a 50 kg person given the maximum recommended dose of 15 mg/kg/day.
14 CLINICAL STUDIES
Bivalirudin Angioplasty Trial (BAT)
In the BAT studies, patients with unstable angina undergoing PCI were randomized 1:1 to a 1 mg/kg bolus of bivalirudin and then 2.5 mg/kg/h for four hours and then 0.2 mg/kg/h for 14 to 20 hours or to 175 IU/kg bolus of heparin followed by an 18- to 24-hour infusion of 15 IU/kg/h infusion. Additional heparin but not bivalirudin could be administered for ACT less than 350 seconds. The studies were designed to demonstrate the superiority of bivalirudin to heparin on the occurrence of any of the following during hospitalization up to seven days of death, MI, abrupt closure of the dilated vessel, or clinical deterioration requiring revascularization or placement of an aortic balloon pump.
The 4312 subjects ranged in age from 29 to 90 (median 63) years. 68% were male, and 91% were Caucasian. Median weight was 80 kg (39 to 120 kg). 741 (17%) subjects had post-MI angina. Twenty-three percent of patients were treated with heparin within one hour prior to randomization.
The studies did not demonstrate that bivalirudin was statistically superior to heparin for reducing the risk of death, MI, abrupt closure of the dilated vessel, or clinical deterioration requiring revascularization or placement of an aortic balloon pump, but the occurrence of these events was similar in both treatment groups. Study outcomes are shown in Table 2.
|
† A composite of death or MI or clinical deterioration of cardiac origin requiring revascularization or placement of an aortic balloon pump or angiographic evidence of abrupt vessel closure. |
||
| Endpoint | Bivalirudin (n=2161) | HEPARIN (n=2151) |
| Primary endpoint† | 7.9% | 9.3% |
| Death, MI, revascularization | 6.2% | 7.9% |
| Death | 0.2% | 0.2% |
| MI | 3.3% | 4.2% |
AT-BAT Trial (NCT# 00043940)
This was a single-arm open-label study in which 51 subjects with heparin-induced thrombocytopenia (HIT) or heparin induced thrombocytopenia and thrombosis syndrome (HITTS) undergoing PCI. The majority of patients achieved adequate ACT at the time of device activation and no major bleeding was reported. Two patients developed thrombocytopenia.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Bivalirudin RTU Injection is supplied as follows:
| NDC | Bivalirudin RTU Injection (5 mg per mL) | Package Factor |
| 70860-403-50 | 250 mg per 50 mL Single-Dose Vial | 1 vial per carton |
| 70860-403-51 | 250 mg per 50 mL Single-Dose Vial | 10 vials per carton |
Bivalirudin RTU Injection is a refrigerated, ready-to-use, clear to slightly opalescent, colorless to yellow, sterile solution.
Each vial contains 250 mg of bivalirudin (equivalent to an average of 275 mg bivalirudin trifluoroacetate*).
*The range of bivalirudin trifluoroacetate is 270 to 280 mg based on a range of trifluoroacetic acid composition of 1.7 to 2.6 equivalents.
16.2 Storage
Store Bivalirudin RTU Injection vials in the refrigerator between 2° to 8°C (36° to 46°F). Excursions are permitted to 20° to 25°C (68° to 77°F) [see Dosage and Administration (2.3)]. Avoid excess heat.
17 PATIENT COUNSELING INFORMATION
PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| BIVALIRUDIN
RTU
bivalirudin injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Athenex Pharmaceutical Division, LLC. (080318964) |