Bone Restore By Life Extension Overview & Drug Interactions
Check For Interactions With Bone Restore
Supplement: Bone Restore by Life Extension
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Vitamin D3
Vitamin D is a fat-soluble vitamin that plays a crucial role in several bodily processes. It helps the body absorb calcium and phosphorus, which are necessary for healthy bones and teeth. It is also important for immune system function and may help to protect against certain diseases. Vitamin D is found in a variety of foods, including fatty fish, egg yolks, and fortified foods such as milk and cereal. It is also produced by the body when the skin is exposed to sunlight. Vitamin D supplements are available in a variety of forms, including tablets, capsules, and liquids. The recommended daily intake of vitamin D varies depending on age, sex, and other factors, and it is important to follow the dosage recommendations provided by a healthcare professional. There are several different forms of vitamin D available, with the two most popular being ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3).
See More Information Regarding Vitamin D| Ingredient Group | Vitamin D |
|---|---|
| Category | vitamin |
- Cholecalciferol
Calcium
Calcium is a vital nutrient found in various foods such as dairy products, certain vegetables, and many fortified items. Over 99% of the body's calcium is stored in the bones and teeth, predominantly as hydroxyapatite. The remaining calcium circulates in the blood, extracellular fluid, muscles, and other tissues, where it is essential for processes like nerve signaling, muscle contraction, vascular activities, glandular secretion, and maintaining cell membrane and capillary permeability. It also plays critical roles in enzyme reactions, respiration, kidney function, and blood clotting, and is involved in neurotransmitter and hormone release, amino acid uptake, vitamin B12 absorption, and gastrin secretion. Calcium balance changes with age: it is positive during periods of growth, stable in adulthood, and tends to become negative in older age. Calcium loss occurs through feces, urine, sweat, and shedding skin cells. In women, reduced estrogen levels decrease calcium absorption and retention, increase bone turnover, and lead to lower bone mass. Calcium supplements come in various forms, including citrate and carbonate, which differ mainly in their calcium content and absorption rates. Calcium citrate is easily absorbed and can be taken without food, making it suitable for older adults or those with low stomach acid. In contrast, calcium carbonate, which contains a higher percentage of calcium, is best absorbed when taken with meals.
See More Information Regarding Calcium| Ingredient Group | Calcium |
|---|---|
| Category | mineral |
- Calcium Fructoborate
- DimaCal Dicalcium Malate
- TRAACS Calcium Bisglycinate Chelate
Magnesium
Magnesium is a mineral that is essential for the proper functioning of the body. It plays a role in many important physiological processes, including the contraction and relaxation of muscles, the transmission of nerve impulses, and the regulation of blood pressure. Magnesium is found in a variety of foods, including leafy green vegetables, nuts, and grains, and it is also available as a dietary supplement. There are several different forms of magnesium that are available as supplements, all of which can be used to prevent deficiency. Additionally, magnesium is purported to have several different health benefits, such as improving sleep, reducing muscle cramps, reducing anxiety, and preventing or treating migraines. Although magnesium is essential for health, magnesium-containing foods and supplements can interact with some prescription medications if used at the same time.
See More Information Regarding Magnesium| Ingredient Group | Magnesium |
|---|---|
| Category | mineral |
- Magnesium Oxide
Zinc
Zinc is a mineral that is essential for the proper functioning of the human body. It is involved in many important physiological processes, including immune system function, wound healing, taste, and smell. Zinc is found in a variety of foods, including meat, seafood, and whole grains, and it is also available as a dietary supplement. Zinc supplements may be used to treat or prevent zinc deficiency, which can occur due to certain medical conditions, such as gastrointestinal disorders, alcohol abuse, and certain medications. Zinc supplements may also be used for other purposes, such as to boost the immune system, improve acne, and reduce the severity and duration of colds. There are several different forms of zinc supplements available, including zinc gluconate, zinc acetate, and zinc sulfate. The most common form of zinc supplements is zinc gluconate, which is well absorbed and is less likely to cause stomach-related side effects than other forms of zinc.
See More Information Regarding Zinc| Ingredient Group | Zinc |
|---|---|
| Category | mineral |
- Zinc Amino Acid Chelate
Manganese
Manganese is an essential trace mineral that plays a vital role in various physiological processes within the human body. It is required for proper brain function, bone formation, enzyme activation, and wound healing. Manganese also contributes to metabolism, antioxidant defense, and the formation of connective tissues.
See More Information Regarding Manganese| Ingredient Group | Manganese |
|---|---|
| Category | mineral |
- Manganese Amino Acid Chelate
Silicon
Silicon, atomic number 14, is the second most abundant element on Earth, after oxygen. It is present in the human body in small amounts (such as in the skin, hair, and nails) and is considered a trace mineral in that regard. Dietary sources of silicon include oats, barley, rice, and some fruits/vegetables. Silicon is a popular dietary supplement, often consumed in the form of silica, which is a chemical compound made up of silicon and oxygen, or as silicic acid (also known as orthosilicic acid). Another common form of silicon utilized is Choline-stabilized orthosilicic acid (ch-OSA), which is the active ingredient in the popular product Biosil. Silicon is used for its purported effects to improve the health and appearance of the skin, hair, and nails. It has also historically been used for other health conditions, such as osteoporosis, cardiovascular disease, and cognitive decline.
See More Information Regarding Silicon| Ingredient Group | Silicon |
|---|---|
| Category | mineral |
- Horsetail extract
Boron
Boron is a trace mineral that is found in a variety of foods, including fruits, vegetables, nuts, and legumes. It is also available as a dietary supplement. Boron is thought to play a role in bone health, as it may help the body metabolize calcium, magnesium, and phosphorus. Some preliminary research also suggests that boron may have anti-inflammatory effects and may help to improve cognitive function. However, more research is needed to confirm these potential benefits. It is important to note that boron is not an essential nutrient and deficiency is rare. The recommended dietary allowance (RDA) for boron is not established, however, the safe upper limit is 20mg/day. Taking high doses of boron can cause skin irritation, nausea, vomiting, and diarrhea. There are several patented forms of boron available in dietary supplements, such as Albion Bororganic Glycine.
See More Information Regarding Boron| Ingredient Group | Boron |
|---|---|
| Category | mineral |
- Calcium Fructoborate
Drugs that interact with Bone Restore by Life Extension
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
Label Information
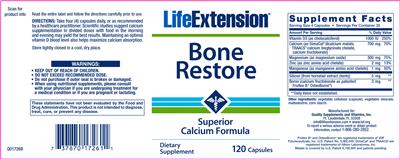
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
4 Capsule(s)
|
| Maximum serving Sizes: |
4 Capsule(s)
|
| Servings per container | 30 |
| UPC/BARCODE | 737870172611 |
| Other Ingredients: |
Vegetable Cellulose
Vegetable Stearate
Maltodextrin
Corn Starch
|
|---|
Label Statments:
| General Statements |
- Scan for product info
- Superior Calcium Formula
- To report a serious adverse event or obtain product information, contact 1-866-280-2852.
|
|---|---|
| Suggested/Recommended/Usage/Directions |
- Read the entire label and follow the directions carefully prior to use.
DIRECTIONS: Take four (4) capsules daily, or as recommended by a healthcare practitioner. Scientific studies suggest calcium supplementation in divided doses with food in the morning and evening may yield the best results. Maintaining an optimal vitamin D blood level also helps maximize calcium absorption.
|
| Storage |
- Store tightly closed in a cool, dry place.
|
| Precautions |
- WARNINGS:
~ KEEP OUT OF REACH OF CHILDREN.
- ~ DO NOT EXCEED RECOMMENDED DOSE.
~ Do not purchase if outer seal is broken or damaged.
- ~ When using nutritional supplements, please consult with your physician if you are undergoing treatment for a medical condition or if you are pregnant or lactating.
|
| FDA Disclaimer Statement |
- These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
|
| FDA Statement of Identity |
- Dietary Supplement
|
| Brand IP Statement(s) |
- Fruitex B and OsteoBoron are registered trademarks of VDF Futureceuticals, Inc. U.S. Patent No. 5,962,049. DimaCal and TRAACS are registered trademarks of Albion Laboratories, Inc.
Malate is covered by U.S. Patent 6,706,904 and patents pending.
|
| General |
- Q01726B
|
Brand Information
See all products by this brand
| Manufactured for: | |
|---|---|
| Name | Quality Supplements and Vitamins, Inc. |
| City | Ft. Lauderdale |
| State | Florida |
| ZipCode | 33309 |
| Phone Number | 1-866-280-2852 |
| [email protected] | |
| Web Address | www.lef.org |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.