Cardio Flow By PL Progressive Laboratories Overview & Drug Interactions
Check For Interactions With Cardio Flow
Supplement: Cardio Flow by PL Progressive Laboratories
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Vitamin A
| Ingredient Group | Vitamin A (unspecified) |
|---|---|
| Category | vitamin |
- Retinol Palmitate
Vitamin C
| Ingredient Group | Vitamin C |
|---|---|
| Category | vitamin |
- Ascorbic Acid
- Selenium Ascorbate
- Zinc Ascorbate
Magnesium
Magnesium is a mineral that is essential for the proper functioning of the body. It plays a role in many important physiological processes, including the contraction and relaxation of muscles, the transmission of nerve impulses, and the regulation of blood pressure. Magnesium is found in a variety of foods, including leafy green vegetables, nuts, and grains, and it is also available as a dietary supplement. There are several different forms of magnesium that are available as supplements, all of which can be used to prevent deficiency. Additionally, magnesium is purported to have several different health benefits, such as improving sleep, reducing muscle cramps, reducing anxiety, and preventing or treating migraines. Although magnesium is essential for health, magnesium-containing foods and supplements can interact with some prescription medications if used at the same time.
See More Information Regarding Magnesium| Ingredient Group | Magnesium |
|---|---|
| Category | mineral |
- Magnesium Aspartate
- Magnesium Orotate
Zinc
Zinc is a mineral that is essential for the proper functioning of the human body. It is involved in many important physiological processes, including immune system function, wound healing, taste, and smell. Zinc is found in a variety of foods, including meat, seafood, and whole grains, and it is also available as a dietary supplement. Zinc supplements may be used to treat or prevent zinc deficiency, which can occur due to certain medical conditions, such as gastrointestinal disorders, alcohol abuse, and certain medications. Zinc supplements may also be used for other purposes, such as to boost the immune system, improve acne, and reduce the severity and duration of colds. There are several different forms of zinc supplements available, including zinc gluconate, zinc acetate, and zinc sulfate. The most common form of zinc supplements is zinc gluconate, which is well absorbed and is less likely to cause stomach-related side effects than other forms of zinc.
See More Information Regarding Zinc| Ingredient Group | Zinc |
|---|---|
| Category | mineral |
- Zinc Ascorbate
Selenium
Selenium is an essential trace element that plays a crucial role in various physiological functions in the human body. It is often included in dietary supplements due to its antioxidant properties and its contribution to maintaining a healthy immune system. Selenium is important for thyroid function and may also have a role in protecting against certain chronic diseases. However, it's essential to note that excessive selenium intake can be harmful, so it's crucial to adhere to recommended daily allowances and consult with a healthcare professional before taking selenium supplements.
See More Information Regarding Selenium| Ingredient Group | Selenium |
|---|---|
| Category | mineral |
- Selenium Ascorbate
Sodium
| Ingredient Group | Sodium |
|---|---|
| Category | mineral |
- Disodium EDTA
Potassium
| Ingredient Group | Potassium |
|---|---|
| Category | mineral |
- Potassium Aspartate
- Potassium Orotate
EDTA
| Ingredient Group | EDTA |
|---|---|
| Category | non-nutrient/non-botanical |
Cardio Flow
| Ingredient Group | Proprietary Blend (Herb/Botanical) |
|---|---|
| Category | blend |
-
Guggul resin extract
Description:Guggal, also known as Commiphora mukul or Indian bdellium, is a species of tree native to India, Pakistan, and parts of the Middle East. It is known for producing a gum resin that has a long history of use in traditional Ayurvedic medicine. Guggul is used for a variety of purposes, including for the treatment of joint pain, obesity, and high cholesterol. Many guggal preparations are often standardized to contain 2.5% to 5% guggulsterone.
See More Information Regarding Guggul
Ingredient Group Guggul Category botanical Forms- Guggulsterones (2.5%)
Bacopin Bacopa leaf extract
Description:Bacopa (Bacopa monnieri) is a perennial herb that is native to India and other parts of Southeast Asia. Bacopa has small, white or blue flowers and is commonly found in wetland areas. In traditional Ayurvedic medicine, bacopa is believed to have a number of health benefits, including the ability to improve memory and cognitive function, reduce anxiety and stress, and improve symptoms of ADHD. It is also thought to have potential benefits for people with Alzheimer's disease and other forms of dementia. Bacopa dietary supplements are often marketed for 'cognitive support'.
See More Information Regarding Bacopa
Ingredient Group Bacopa Category botanical Forms- Bacosides (20%)
Arjuna bark extract
Description:Terminalia is a genus of trees that are native to tropical and subtropical regions of the world. There are around 100 species of Terminalia, which are known for their hard, durable wood and their ability to grow in a variety of climates. In traditional medicine, three species are of particular note, Terminalia arjuna, Terminalia bellirica, and Terminalia chebula. Various parts of these trees, including the bark, leaves, and fruit, are used as a natural remedy for digestive problems, such as constipation and diarrhea, and to boost the immune system. They are also believed to have anti-inflammatory properties, and they may be helpful in reducing inflammation and swelling in the body.
See More Information Regarding Terminalia
Ingredient Group Terminalia Category botanical Forms- Arjunolic Acid (0.5%)
Indian Elecampane root extract
Description:Elecampane is a perennial herb that is native to Europe and Asia. It has been used in traditional medicine for centuries to treat respiratory conditions, such as asthma, bronchitis, and coughs. The roots and rhizomes of the plant are used to make medicine. Preliminary research suggests that compounds in elecampane may have expectorant, bronchodilator, and anti-inflammatory properties, which may help to relieve symptoms of respiratory disorders.
See More Information Regarding Elecampane
Ingredient Group Elecampane Category botanical Forms- Alantolactone (2%)
- Isoalantolactone
Cilantro
Coriander, also known as cilantro, is an herb in the Apiaceae family. Both the fruit (seed) and leaves of the coriander plant are used in food as a spice and in traditional medicine. It is native to the Mediterranean region and is widely cultivated in many parts of the world. When used as medicine, coriander is most often used for gastrointestinal problems, including dyspepsia, nausea, diarrhea, flatulence, constipation, irritable bowel syndrome (IBS), and to stimulate the appetite. Coriander, when used topically, is also thought to have antimicrobial effects.
See More Information Regarding Coriander| Ingredient Group | Coriander |
|---|---|
| Category | botanical |
Bromelain
Bromelain is a group of proteolytic enzymes derived from pineapples. It has been traditionally used for its anti-inflammatory and protein-digesting properties. Some research suggests it may have potential benefits for conditions such as allergic rhinitis, burns, cancer, diabetic foot ulcers, and postoperative pain, among others. However, the evidence for its effectiveness in these areas is lacking, and more research is needed. It is important to note that bromelain should not be confused with other protein-digesting enzymes. Pregnant and lactating individuals should avoid using bromelain due to insufficient information about its safety in these populations.
See More Information Regarding Bromelain| Ingredient Group | Bromelain |
|---|---|
| Category | enzyme |
Disodium EDTA
| Ingredient Group | EDTA |
|---|---|
| Category | non-nutrient/non-botanical |
Butcher's Broom rhizome extract
Butcher's broom (Ruscus aculeatus) is a plant species in the lily family that is native to Europe and parts of Asia. It is a low-growing evergreen shrub with small, spiky leaves, clusters of small, greenish flowers and small red berries. Butcher's broom, specifically the rhizomes (underground stems) and roots, has been used in traditional medicine to treat a variety of conditions, including circulation problems, kidney issues, and varicose veins. It is thought to have anti-inflammatory, laxative and vasoconstrictive properties and is a popular dietary supplement, often marketed to improve circulation and reduce swelling.
See More Information Regarding Butcher's Broom| Ingredient Group | Butcher's Broom |
|---|---|
| Category | botanical |
- Ruscogenins (10%)
Papain
Papain is a proteolytic enzyme that is extracted from the papaya fruit. It is also known as papaya proteinase. Proteolytic enzymes are enzymes that break down proteins into smaller peptides and amino acids. Papain specifically breaks down proteins by hydrolyzing the peptide bonds that link amino acids together. Papain is used in a variety of applications, including food production, medicine, and biotechnology. Medically, papain can be used to remove dead tissue from wounds and to treat certain skin conditions. It has also been used to treat sports injuries, such as sprains and strains. Additionally, it is a common ingredient in digestive enzyme dietary supplements to aid in the digestion of proteins.
See More Information Regarding Papain| Ingredient Group | Papain |
|---|---|
| Category | enzyme |
L-Glutathione
Glutathione is a naturally occurring compound that is composed of three amino acids: cysteine, glutamic acid, and glycine. It is found in nearly all cells in the body and plays a crucial role in many physiological processes, including as an antioxidant defense, detoxification, and immune function. Glutathione levels in the body can be decreased by a number of factors, such as aging, stress, poor diet, and exposure to toxins. Some people take glutathione supplements in the hopes of increasing their levels and potentially benefiting from its antioxidant properties. Glutathione supplements are available in various forms, including oral supplements, intravenous (IV) injections, and nebulized (inhaled) forms. Oral glutathione supplements are often in a liposomal form to improve absorption.
See More Information Regarding Glutathione| Ingredient Group | Glutathione |
|---|---|
| Category | non-nutrient/non-botanical |
Drugs that interact with Cardio Flow by PL Progressive Laboratories
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
Label Information
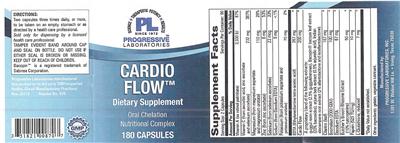
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
2 Capsule(s)
|
| Maximum serving Sizes: |
2 Capsule(s)
|
| Servings per container | 90 |
| UPC/BARCODE | 351821008707 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Vitamin A |
3330 IU
|
Vitamin A (unspecified) |
67%
|
| Vitamin C |
232 mg
|
Vitamin C |
387%
|
| Magnesium |
110 mg
|
Magnesium |
28%
|
| Zinc |
8 mg
|
Zinc |
53%
|
| Selenium |
23 mcg
|
Selenium |
33%
|
| Sodium |
9 mg
|
Sodium |
1%
|
| Potassium |
70 mg
|
Potassium |
2%
|
| EDTA |
267 mg
|
EDTA |
--
|
| Cardio Flow |
200 mg
|
Proprietary Blend (Herb/Botanical) |
--
|
| Guggul resin extract |
0 NP
|
Guggul |
|
| Bacopin Bacopa leaf extract |
0 NP
|
Bacopa |
|
| Arjuna bark extract |
0 NP
|
Terminalia |
|
| Indian Elecampane root extract |
0 NP
|
Elecampane |
|
| Cilantro |
167 mg
|
Coriander |
--
|
| Bromelain |
100 mg
|
Bromelain |
--
|
| Disodium EDTA |
67 mg
|
EDTA |
--
|
| Butcher's Broom rhizome extract |
50 mg
|
Butcher's Broom |
--
|
| Papain |
10 mg
|
Papain |
--
|
| L-Glutathione |
7 mg
|
Glutathione |
--
|
| Other Ingredients: |
Gelatin
Vegetable Lubricant
|
|---|
Label Statments:
| Suggested/Recommended/Usage/Directions |
- Directions: Two capsules three times daily, or more, to be taken on an empty stomach or as directed by a health care professional.
|
|---|---|
| General Statements |
- Sold only for dispensing by a licensed health care professional.
- Progressive Laboratories manufactured this product in its 3rd party GMP-inspected facility. (Good Manufacturing Practices)
- Science + therapeutic potency = results
PL
Since 1972
- Oral chelation
Nutritional complex
|
| Precautions |
- Tamper evident band around cap and seal on bottle. Do not use if either seal is broken or missing.
- Keep out of reach of children.
|
| Brand IP Statement(s) |
- Bacopin is a registered trademark of Sabinsa Corperatioin.
|
| General |
- Rev. J0113 Reorder No. 870
|
| Seals/Symbols |
- 3rd party confirmed GMP compliant
|
| FDA Statement of Identity |
- Dietary Supplement
|
Brand Information
See all products by this brand
| Manufactured by: | |
|---|---|
| Name | Progressive Laboratories, Inc. |
| Street Address | 1701 W. Walnut Hill Ln. |
| City | Irving |
| State | Texas |
| ZipCode | 75038 |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.