Feminene Female Support Formula By Market America Overview & Drug Interactions
Check For Interactions With Feminene Female Support Formula
Supplement: Feminene Female Support Formula by Market America
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Vitamin E
Vitamin E is a fat-soluble vitamin found naturally in a variety of foods, including vegetable oils, nuts, seeds, and leafy green vegetables. Vitamin E is also available as a dietary supplement and is often added to skincare products. Vitamin E has many roles in the body, including protecting cells from oxidative stress and supporting immune function.
See More Information Regarding Vitamin E| Ingredient Group | Vitamin E |
|---|---|
| Category | vitamin |
- D-Alpha-Tocopheryl Succinate
Thiamine Hydrochloride
| Ingredient Group | Thiamin |
|---|---|
| Category | vitamin |
- Vitamin B1
Riboflavin
| Ingredient Group | Riboflavin |
|---|---|
| Category | vitamin |
- Vitamin B2
Niacin
Niacin, also known as vitamin B3, is a water-soluble vitamin found in a variety of foods, including meat, poultry, fish, and fortified grains. It plays a variety of roles in the body, including in the metabolism of carbohydrates, fats, and proteins. It is also necessary for the production of energy in the body and in the synthesis of different hormones. Niacin deficiency is rare in developed countries, however, supplementation has been shown to have several positive benefits. For example, it can reduce the risk of diabetic neuropathy (i.e., nerve pain) and has been shown effective for treating some types of high cholesterol (extended-release niacin is used as a prescription drug in the United States for this purpose). There is a multitude of niacin forms available as dietary supplements, including NADH, niacinamide, and nicotinamide riboside, all with different properties.
See More Information Regarding Niacin| Ingredient Group | Niacin |
|---|---|
| Category | vitamin |
- Vitamin B3
Vitamin B6
| Ingredient Group | Vitamin B6 |
|---|---|
| Category | vitamin |
- Pyridoxine Hydrochloride
Folate
| Ingredient Group | Folate |
|---|---|
| Category | vitamin |
- Folic Acid
Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin crucial for several bodily functions. It plays a pivotal role in the formation of red blood cells, aiding in the prevention of anemia. Vitamin B12 is essential for maintaining a healthy nervous system and proper brain function, as it is involved in the synthesis of myelin, the protective sheath around nerve fibers. This vitamin is primarily found in animal-based foods such as meat, fish, dairy products, and eggs, making it important for vegetarians and vegans to consider supplementation. A deficiency in vitamin B12 can lead to neurological issues, fatigue, and cognitive impairment.
See More Information Regarding Vitamin B12| Ingredient Group | Vitamin B12 |
|---|---|
| Category | vitamin |
- Cyanocobalamin
Calcium D-Pantothenate
Calcium is a vital nutrient found in various foods such as dairy products, certain vegetables, and many fortified items. Over 99% of the body's calcium is stored in the bones and teeth, predominantly as hydroxyapatite. The remaining calcium circulates in the blood, extracellular fluid, muscles, and other tissues, where it is essential for processes like nerve signaling, muscle contraction, vascular activities, glandular secretion, and maintaining cell membrane and capillary permeability. It also plays critical roles in enzyme reactions, respiration, kidney function, and blood clotting, and is involved in neurotransmitter and hormone release, amino acid uptake, vitamin B12 absorption, and gastrin secretion. Calcium balance changes with age: it is positive during periods of growth, stable in adulthood, and tends to become negative in older age. Calcium loss occurs through feces, urine, sweat, and shedding skin cells. In women, reduced estrogen levels decrease calcium absorption and retention, increase bone turnover, and lead to lower bone mass. Calcium supplements come in various forms, including citrate and carbonate, which differ mainly in their calcium content and absorption rates. Calcium citrate is easily absorbed and can be taken without food, making it suitable for older adults or those with low stomach acid. In contrast, calcium carbonate, which contains a higher percentage of calcium, is best absorbed when taken with meals.
See More Information Regarding Calcium| Ingredient Group | Calcium |
|---|---|
| Category | mineral |
- Vitamin B5
Soy Extract
| Ingredient Group | Soy |
|---|---|
| Category | botanical |
Dong Quai
Dong quai (Angelica sinensis) is a plant native to Asia and has been used in traditional medicine for centuries. It is often referred to as "female ginseng" and is used to treat a variety of health conditions, particularly those related to the female reproductive system. It is also claimed to have anti-inflammatory and antioxidant properties as well as an ability to improve blood circulation. When utilized for dietary supplements, the root of Dong quai is used.
See More Information Regarding Dong Quai| Ingredient Group | Dong Quai |
|---|---|
| Category | botanical |
Evening Primrose Oil
Evening primrose (Oenothera biennis) is a plant native to North and Central America and a member of the Onagraceae family. It is an annual plant that has bright yellow flowers that bloom in the evening (hence the name). The plant produces seeds that are rich in a fatty acid called gamma-linolenic acid (GLA). Evening primrose oil is a dietary supplement that is derived from the seeds of the evening primrose plant. It is rich in GLA (gamma-linolenic acid), an omega-6 fatty acid, and is sometimes taken to improve the health of the skin, reduce inflammation, and reduce the symptoms of premenstrual syndrome (PMS). It has also been used historically for alcohol use disorder. Dietary supplements containing evening primrose oil generally are standardized by their gamma-linolenic acid (GLA) and linoleic acid (LA) content.
See More Information Regarding Evening Primrose| Ingredient Group | Evening Primrose oil |
|---|---|
| Category | fat |
Wild Yam
| Ingredient Group | Wild Yam |
|---|---|
| Category | botanical |
Black Cohosh root extract
Black cohosh (Actaea racemosa) is a perennial plant native to North America and is sometimes referred to as "black snakeroot" or "bugbane". Black cohosh is often taken as a supplement to help with menopause symptoms, such as hot flashes, night sweats, and mood changes. It is also sometimes used to help with premenstrual syndrome (PMS) and other menstrual issues. Black cohosh supplements are typically standardized for their triterpene glycoside content.
See More Information Regarding Black Cohosh| Ingredient Group | Black Cohosh |
|---|---|
| Category | botanical |
Chasteberry extract
| Ingredient Group | Chastetree |
|---|---|
| Category | botanical |
Horsetail
Horsetail, also known as equisetum, is a type of perennial herb that belongs to the Equisetaceae family. It is native to much of the Northern Hemisphere and has distinctive, jointed stems that resemble the tail of a horse, hence common name. Some species of horsetail are used medicinally and have been traditionally used to treat a range of ailments, including kidney and bladder problems, wounds, and hair loss. It is most commonly used in traditional medicine as an oral diuretic (i.e., water pill) for the treatment of edema. It is important to note that some species of horsetail (e.g., Equisetum palustre) may be toxic and should not be consumed. Additionally, some types of horsetail contain thiaminase, which can cause thiamine deficiency with prolonged use.
See More Information Regarding Horsetail| Ingredient Group | Horsetail |
|---|---|
| Category | botanical |
Red Clover
Red clover (Trifolium pratense) is a perennial plant that is native to Europe and Asia, but is also found in many parts of the world, including North America. Red clover has a long history of use in traditional medicine and is believed to have a number of potential health benefits. It is a rich source of isoflavones, which are thought to have estrogen-like effects and is therefore often marketed for women's health. It has been used to treat hot flashes and other menopause symptoms as well as cardiovascular disease, high cholesterol, and osteoporosis.
See More Information Regarding Red Clover| Ingredient Group | Red Clover |
|---|---|
| Category | botanical |
Passiflora
| Ingredient Group | Passiflora (unspecified) |
|---|---|
| Category | botanical |
Valerian
Valerian (Valeriana officinalis) is a perennial herb native to Europe and Asia. The plant is known for its strong, distinctive odor and its purported medicinal effects. Valerian contains a number of active compounds, including valerenic acid and valepotriates, which are believed to have a sedative effect on the body and are may to be helpful in the treatment of insomnia and anxiety. Valerian is also believed to have mild tranquilizing and muscle-relaxing properties and may be helpful in the treatment of muscle spasms and other muscle disorders. The root of the valerian plant is most commonly utilized in dietary supplements and is often standardized for valerenic acid content.
See More Information Regarding Valerian| Ingredient Group | Valerian |
|---|---|
| Category | botanical |
Sage
Sage (Salvia officinalis) is a perennial herb native to the Mediterranean region. It is a member of the mint family (Lamiaceae) and is widely cultivated for its fragrant leaves and its culinary uses. Sage has a long history of use in traditional medicine and is purported to have a number of potential health benefits. It is thought to have antioxidant, anti-inflammatory, and antimicrobial properties, and it is used to treat a variety of conditions, including digestive disorders, respiratory conditions, and skin problems. Sage is also sometimes used as a natural remedy for anxiety and stress.
See More Information Regarding Sage| Ingredient Group | Sage |
|---|---|
| Category | botanical |
St. John's Wort
St. John's wort (Hypericum perforatum) is a plant native to Europe and is known for its medicinal properties. St. John's wort has been used to treat a variety of conditions, including depression, anxiety, and sleep disorders. It is one of the most extensively used and researched natural medicines in the world, and is thought to work similarly to certain conventional antidepressants by increasing the levels of serotonin in the brain.
See More Information Regarding St. John's Wort| Ingredient Group | St. John's Wort |
|---|---|
| Category | botanical |
Drugs that interact with Feminene Female Support Formula by Market America
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
Label Information
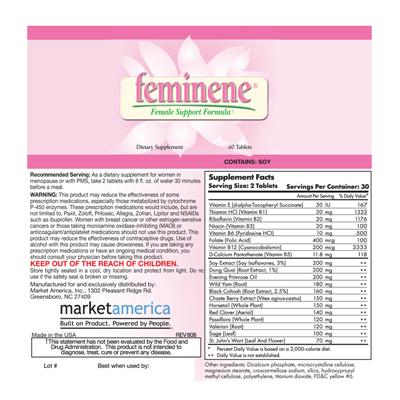
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
2 Tablet(s)
|
| Maximum serving Sizes: |
2 Tablet(s)
|
| Servings per container | 30 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Vitamin E |
50 IU
|
Vitamin E |
167%
|
| Thiamine Hydrochloride |
20 mg
|
Thiamin |
1333%
|
| Riboflavin |
20 mg
|
Riboflavin |
1176%
|
| Niacin |
20 mg
|
Niacin |
100%
|
| Vitamin B6 |
10 mg
|
Vitamin B6 |
500%
|
| Folate |
400 mcg
|
Folate |
100%
|
| Vitamin B12 |
200 mcg
|
Vitamin B12 |
3333%
|
| Calcium D-Pantothenate |
11.8 mg
|
Calcium |
118%
|
| Soy Extract |
200 mg
|
Soy |
--
|
| Dong Quai |
200 mg
|
Dong Quai |
--
|
| Evening Primrose Oil |
200 mg
|
Evening Primrose oil |
--
|
| Wild Yam |
180 mg
|
Wild Yam |
--
|
| Black Cohosh root extract |
160 mg
|
Black Cohosh |
--
|
| Chasteberry extract |
150 mg
|
Chastetree |
--
|
| Horsetail |
150 mg
|
Horsetail |
--
|
| Red Clover |
140 mg
|
Red Clover |
--
|
| Passiflora |
120 mg
|
Passiflora (unspecified) |
--
|
| Valerian |
120 mg
|
Valerian |
--
|
| Sage |
100 mg
|
Sage |
--
|
| St. John's Wort |
70 mg
|
St. John's Wort |
--
|
| Other Ingredients: |
Dicalcium Phosphate
Microcrystalline Cellulose
Magnesium Stearate
Croscarmellose Sodium
Silica
Hydroxypropyl Methyl Cellulose
Polyethylene
Titanium Dioxide
Titanium Dioxide
FD&C Yellow #6
|
|---|
Label Statments:
| FDA Statement of Identity |
- Dietary Supplement
|
|---|---|
| Suggested/Recommended/Usage/Directions |
- Recommended Serving: As a dietary supplement for women in menopause or with PMS, take 2 tablets with 8 fl. oz. of water 30 minutes before a meal.
|
| Precautions |
- KEEP OUT OF THE REACH OF CHILDREN.
- Do not use if the safety seal is broken or missing.
- WARNING: This product may reduce the effectiveness of some prescription medications, especially those metabolized by cytochrome P-450 enzymes. These prescription medications would include, but are not limited to, Paxil, Zoloft, Prilosec, Allegra, Zofran, Lipitor, and NSAIDs such as ibuprofen. Women with breast cancer or other estrogen-sensitive cancers or those taking monoamine oxidase-inhibiting (MAOI) or anticoagulant/antiplatelet medications should not use this product. This product may reduce the effectiveness of contraceptive drugs. Use of alcohol with this product may cause drowsiness. If you are taking any prescription medications or have an ongoing medical condition, you should consult your physician before taking this product.
|
| FDA Disclaimer Statement |
- †This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
|
| Storage |
- Store tightly sealed in a cool, dry location and protect from light.
|
| Formula |
- CONTAINS: SOY
|
| General Statements |
- Lot #: Best when used by:
- Made in the USA
- marketamerica Built on Product. Powered by People.
|
| General |
- REV 808
|
Brand Information
See all products by this brand
| Manufactured for and exclusively distributed by: | |
|---|---|
| Name | Market America, Inc. |
| Street Address | 1302 Pleasant Ridge Rd. |
| City | Greensboro |
| State | NC |
| ZipCode | 27409 |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.