PhytoCort By Allergy Research Group Overview & Drug Interactions
Check For Interactions With PhytoCort
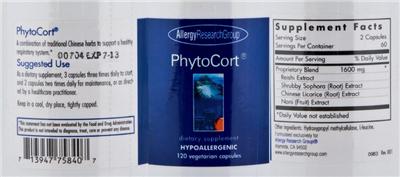
Supplement: PhytoCort by Allergy Research Group
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Proprietary Blend
| Ingredient Group | Proprietary Blend (Combination) |
|---|---|
| Category | blend |
-
Reishi extract
Description:Reishi mushroom (Ganoderma lucidum) is a type of mushroom native to Asia. It is a relatively tough mushroom with a shiny, reddish-brown exterior and a bitter, woody taste. Reishi mushroom is popular in traditional medicine, with a history of use dating back several thousand years. It is purported to have a number of health benefits, including the ability to boost the immune system, reduce inflammation, and improve overall well-being.
See More Information Regarding Reishi Mushroom
Ingredient Group Reishi mushroom Category botanical
Shrubby Sophora (root) extract
Ingredient Group Shrubby Sophora Category botanical
Chinese Licorice (root) extract
Description:Licorice, also known as glycyrrhiza, is a plant native to parts of Europe, Asia, and the Mediterranean region. The root of the plant has been used for centuries in traditional medicine and is often consumed in the form of candy, tea, and supplements. Licorice has a number of purported health benefits, including the ability to soothe sore throats, reduce inflammation, and improve digestive problems. It may also have antiviral and antibacterial properties. Licorice naturally contains glycyrrhizin, or glycyrrhizic acid, which can have toxic effects if consumed in large amounts. However, licorice can be processed to remove glycyrrhizin, resulting in DGL (deglycyrrhizinated licorice). Many dietary supplements that contain deglycyrrhizinated licorice are often simply named 'DGL'.
See More Information Regarding Licorice
Ingredient Group Chinese Licorice Category botanical
Noni (fruit) extract
Description:Noni (Morinda citrifolia) is a tropical fruit that is native to Southeast Asia and the Pacific Islands. It is also known by a variety of other names, including Indian mulberry, beach mulberry, and cheese fruit. Noni fruit is typically not eaten fresh due to its strong flavor and smell. Regardless, many parts of the plant, including the roots, fruit, stem, bark, leaves, and flowers are used to make juice, supplements, and other products. Noni has a long history of use in traditional medicine. Some studies have suggested that noni may have potential health benefits, including the ability to reduce inflammation, improve immune function, and lower blood pressure. It is also thought to have antioxidant and anti-tumor effects.
See More Information Regarding Noni
Ingredient Group Noni Category botanical
Drugs that interact with PhytoCort by Allergy Research Group
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
Label Information
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
3 Capsule(s)
|
| Maximum serving Sizes: |
3 Capsule(s)
|
| Servings per container | 60 |
| UPC/BARCODE | 713947758407 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Proprietary Blend |
1600 mg
|
Proprietary Blend (Combination) |
--
|
| Reishi extract |
0 NP
|
Reishi mushroom |
|
| Shrubby Sophora (root) extract |
0 NP
|
Shrubby Sophora |
|
| Chinese Licorice (root) extract |
0 NP
|
Chinese Licorice |
|
| Noni (fruit) extract |
0 NP
|
Noni |
|
| Other Ingredients: |
Hydroxypropyl Methylcellulose
L-Leucine
|
|---|
Label Statments:
| General Statements |
- A combination of traditional Chinese herbs to support a healthy respiratory system.*
00704 EXP 7-13
|
|---|---|
| Suggested/Recommended/Usage/Directions |
- Suggested Use
As a dietary supplement, 3 capsules three times daily to start, and 2 capsules two times daily for maintenance, or as directed by a healthcare practitioner.
|
| Storage |
- Keep in a cool, dry place, tightly capped.
|
| FDA Disclaimer Statement |
- This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
|
| FDA Statement of Identity |
- dietary supplement
|
| Formula |
- HYPOALLERGENIC
|
| General |
- Rev. 001
|
Brand Information
See all products by this brand
| Formulated exclusively for | |
|---|---|
| Name | Allergy Research Group |
| City | Alameda |
| State | CA |
| ZipCode | 94502 |
| Web Address | www.allergyresearchgroup.com |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.