Super Carnosine 500 mg By Life Extension Overview & Drug Interactions
Check For Interactions With Super Carnosine 500 mg
Supplement: Super Carnosine 500 mg by Life Extension
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Thiamine
| Ingredient Group | Thiamin |
|---|---|
| Category | vitamin |
- Vitamin B1
Carnosine
| Ingredient Group | Carnosine |
|---|---|
| Category | non-nutrient/non-botanical |
Benfotiamine
Benfotiamine is a synthetic derivative of thiamine (vitamin B1) with enhanced bioavailability. It is often used as a dietary supplement to support overall health and address conditions related to thiamine deficiency. Unlike thiamine, benfotiamine is lipid-soluble, allowing it to penetrate cell membranes more effectively. This enhanced absorption is believed to contribute to its potential therapeutic benefits in managing diabetic complications, neuropathy, and oxidative stress.
See More Information Regarding Benfotiamine| Ingredient Group | Benfotiamine |
|---|---|
| Category | vitamin |
Luteolin
| Ingredient Group | Luteolin |
|---|---|
| Category | non-nutrient/non-botanical |
- Perilla leaf extract
Drugs that interact with Super Carnosine 500 mg by Life Extension
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
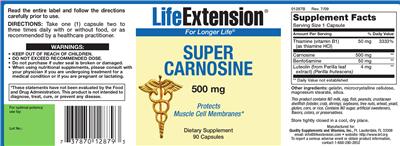
Label Information
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
1 Capsule(s)
|
| Maximum serving Sizes: |
1 Capsule(s)
|
| UPC/BARCODE | 737870128793 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Thiamine |
50 mg
|
Thiamin |
3333%
|
| Carnosine |
500 mg
|
Carnosine |
--
|
| Benfotiamine |
50 mg
|
Benfotiamine |
--
|
| Luteolin |
4 mg
|
Luteolin |
--
|
| Other Ingredients: |
Gelatin
Microcrystalline Cellulose
Magnesium Stearate
Silica
|
|---|
Label Statments:
| Suggested/Recommended/Usage/Directions |
- DIRECTIONS: Take one (1) capsule two to three times daily with or without food, or as recommended by a healthcare practitioner.
|
|---|---|
| Precautions |
- WARNINGS: - KEEP OUT OF REACH OF CHILDREN.
- - When using nutritional supplements, please consult with your physician if you are undergoing treatment for a medical condition or if you are pregnant or lactating.
- - DO NOT EXCEED RECOMMENDED DOSE.
- - Do not purchase if outer seal is broken or damaged.
|
| FDA Disclaimer Statement |
- *These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
|
| Storage |
- Store tightly closed in a cool, dry place.
|
| General |
- 01287B Rev. 7/09
|
| General Statements |
- 500 mg
- Protects Muscle Cell Membranes*
- Read the entire label and follow the directions carefully prior to use.
|
| Brand IP Statement(s) |
- For Longer Life(R)
|
| Formulation |
- This product contains NO milk, egg, fish, peanuts, crustacean shellfish (lobster, crab, shrimp), soybeans, tree nuts, wheat, yeast, gluten, corn, or rice. Contains NO sugar, artificial sweeteners, flavors, colors, or preservatives.
|
| FDA Statement of Identity |
- Dietary Supplement
|
Brand Information
See all products by this brand
| Manufactured for: | |
|---|---|
| Name | Quality Supplements and Vitamins, Inc. |
| City | Ft. Lauderdale |
| State | FL |
| ZipCode | 33309 |
| Phone Number | 1-888-280-2852 |
| [email protected] | |
| Web Address | http://www.lef.org |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.