Water Pill By GNC BodyDynamix Overview & Drug Interactions
Check For Interactions With Water Pill
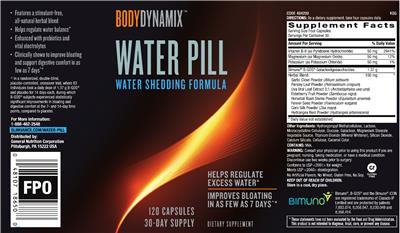
Supplement: Water Pill by GNC BodyDynamix
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Vitamin B6
| Ingredient Group | Vitamin B6 |
|---|---|
| Category | vitamin |
- Pyridoxine Hydrochloride
Magnesium
Magnesium is a mineral that is essential for the proper functioning of the body. It plays a role in many important physiological processes, including the contraction and relaxation of muscles, the transmission of nerve impulses, and the regulation of blood pressure. Magnesium is found in a variety of foods, including leafy green vegetables, nuts, and grains, and it is also available as a dietary supplement. There are several different forms of magnesium that are available as supplements, all of which can be used to prevent deficiency. Additionally, magnesium is purported to have several different health benefits, such as improving sleep, reducing muscle cramps, reducing anxiety, and preventing or treating migraines. Although magnesium is essential for health, magnesium-containing foods and supplements can interact with some prescription medications if used at the same time.
See More Information Regarding Magnesium| Ingredient Group | Magnesium |
|---|---|
| Category | mineral |
- Magnesium Oxide
Potassium
| Ingredient Group | Potassium |
|---|---|
| Category | mineral |
- Potassium Chloride
Bimuno B-GOS Galactooligosaccharides
| Ingredient Group | Galactooligosaccharides |
|---|---|
| Category | complex carbohydrate |
Herbal Blend
| Ingredient Group | Blend (Herb/Botanical) |
|---|---|
| Category | blend |
-
Garlic clove powder
Description:Garlic (Allium sativum) is a popular culinary herb native to central Asia, but is now widely cultivated. It is a member of the onion family and is known for its pungent smell and flavor. In addition to its culinary use, it has been used medicinally to treat a variety of conditions, including high blood pressure, high cholesterol, and infections.
See More Information Regarding Garlic
Ingredient Group Garlic Category botanical
Parsley leaf powder
Description:Parsley is a herb that is native to the Mediterranean region and is widely cultivated for its leaves, which are used as a spice in cooking. In traditional medicine, parsley is believed to have a number of health benefits and is often used as a natural diuretic, increasing urine production. This property may make parsley useful in the treatment of certain conditions, such as bloating and water retention. It is also commonly used for gastrointestinal problems.
See More Information Regarding Parsley
Ingredient Group Parsley Category botanical
Uva Ursi leaf extract
Description:Uva ursi, also known as bearberry, is a plant species belonging to the Ericacea family, and is native to North America, Europe, and parts of Asia. It is a low-growing shrub with small, white flowers and edible red berries. The leaves of the plant are often used in traditional medicine as they are thought to have various medicinal properties, such as astringent, diuretic, and antimicrobial effects. Medicinally, Uva ursi is most often used to treat urinary tract infections and other urinary problems, as well as skin conditions such as eczema and dermatitis. It is also sometimes used to promote weight loss and as a natural remedy for kidney stones. In dietary supplements, Uva ursi is often standardized with a specific amount of Arbutin (usually around 20%), a constituent of the plant.
See More Information Regarding Uva Ursi
Ingredient Group Uva Ursi Category botanical
Elderberry fruit powder
Ingredient Group European Elder Category botanical
Horsetail rush stems powder
Description:Horsetail, also known as equisetum, is a type of perennial herb that belongs to the Equisetaceae family. It is native to much of the Northern Hemisphere and has distinctive, jointed stems that resemble the tail of a horse, hence common name. Some species of horsetail are used medicinally and have been traditionally used to treat a range of ailments, including kidney and bladder problems, wounds, and hair loss. It is most commonly used in traditional medicine as an oral diuretic (i.e., water pill) for the treatment of edema. It is important to note that some species of horsetail (e.g., Equisetum palustre) may be toxic and should not be consumed. Additionally, some types of horsetail contain thiaminase, which can cause thiamine deficiency with prolonged use.
See More Information Regarding Horsetail
Ingredient Group Horsetail Category botanical
Fennel seed powder
Description:Fennel is a plant species in the carrot family and native to the Mediterranean region. Fennell seeds are often used as a spice, and other parts as food. Fennel has a distinctive, sweet, licorice-like flavor and is often used in cooking to add flavor to dishes such as sauces, salads, and baked goods. It is used in traditional medicine as a good source of vitamins and minerals, such as potassium, beta-carotene and vitamin C. It has also been used to treat a variety of conditions, including digestive issues, respiratory problems, and skin conditions.
See More Information Regarding Fennel
Ingredient Group Fennel Category botanical
Corn silk powder
Ingredient Group Corn Category botanical
Hydrangea root powder
Ingredient Group Hydrangea Category botanical
Drugs that interact with Water Pill by GNC BodyDynamix
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
Label Information
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
4 Capsule(s)
|
| Maximum serving Sizes: |
4 Capsule(s)
|
| Servings per container | 30 |
| UPC/BARCODE | 048107186500 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Vitamin B6 |
50 mg
|
Vitamin B6 |
2941%
|
| Magnesium |
50 mg
|
Magnesium |
12%
|
| Potassium |
50 mg
|
Potassium |
1%
|
| Bimuno B-GOS Galactooligosaccharides |
1.37 Gram(s)
|
Galactooligosaccharides |
--
|
| Herbal Blend |
100 mg
|
Blend (Herb/Botanical) |
--
|
| Garlic clove powder |
0 NP
|
Garlic |
|
| Parsley leaf powder |
0 NP
|
Parsley |
|
| Uva Ursi leaf extract |
0 NP
|
Uva Ursi |
|
| Elderberry fruit powder |
0 NP
|
European Elder |
|
| Horsetail rush stems powder |
0 NP
|
Horsetail |
|
| Fennel seed powder |
0 NP
|
Fennel |
|
| Corn silk powder |
0 NP
|
Corn |
|
| Hydrangea root powder |
0 NP
|
Hydrangea |
|
| Other Ingredients: |
Hydroxypropyl Methylcellulose
Lactose
Microcrystalline Cellulose
Glucose
Galactose
Magnesium Stearate
Titanium Dioxide
Silicon Dioxide
Calcium Silicate
Cellulose
Caramel
|
|---|
Label Statments:
| Formulation |
- Features a stimulant-free, all-natural herbal blend
- No Artificial Flavors, No Wheat, Gluten Free, No Soy.
|
|---|---|
| General Statements |
- Helps regulate water balance
Enhance with prebiotics and vital electrolytes
Clinically shown to improve bloating and support digestive comfort in as few as 7 days
In a randomized, double-blind, placebo-controlled, crossover trial, where 83 individuals took a daily dose of 1.37 g B-GOS and placebo for 14 days each, during which B-GOS subjects experienced statistically significant improvements in bloating and digestive comfort at the 7- and 14-day time points, compared to placebo.
- Water shedding formula
30-day supply
Helps regulate excess water
Improves bloating in as few as 7 days
- Conforms to USP <2091> for weight.
Meets USP <2040> disintegration.
|
| FDA Statement of Identity |
- Dietary Supplement
|
| Suggested/Recommended/Usage/Directions |
- Directions: As a dietary supplement, take four capsules daily.
|
| Formula |
- Contains: Milk.
|
| Precautions |
- Contains: Milk.
- Warning: Consult your physician prior to using this product if you are pregnant, nursing, taking medication, or have a medical condition.
- Discontinue use two weeks prior to surgery.
- Keep out of reach of children.
|
| Seals/Symbols |
- Gf
Gluten free
|
| Storage |
- Store in a cool, dry place.
|
| Brand IP Statement(s) |
- Bimuno, B-GOS and the Bimuno Icon are registered trademarks of Clasado IP Limited and are protected by patents 7,883,874, 8,058,047, 8,030,049 and 8,168,414.
|
| FDA Disclaimer Statement |
- These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
|
Brand Information
See all products by this brand
| Distributed by | |
|---|---|
| Name | General Nutrition Corporation |
| City | Pittsburgh |
| State | PA |
| Country | USA |
| ZipCode | 15222 |
| Phone Number | 1-888-462-2548 |
| Web Address | Slimvance.com/water-pill |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.