Women's Daily Multivitamin By up&up Overview & Drug Interactions
Check For Interactions With Women's Daily Multivitamin
Supplement: Women's Daily Multivitamin by up&up
This product contains
Below is a list of the 'active' ingredients listed on the supplement label for this product.
For a list of 'other ingredients', such as fillers, please see the 'Label Information' section on this page.
Vitamin A
| Ingredient Group | Vitamin A |
|---|---|
| Category | vitamin |
- Beta-Carotene
Vitamin C
| Ingredient Group | Vitamin C |
|---|---|
| Category | vitamin |
Vitamin D
Vitamin D is a fat-soluble vitamin that plays a crucial role in several bodily processes. It helps the body absorb calcium and phosphorus, which are necessary for healthy bones and teeth. It is also important for immune system function and may help to protect against certain diseases. Vitamin D is found in a variety of foods, including fatty fish, egg yolks, and fortified foods such as milk and cereal. It is also produced by the body when the skin is exposed to sunlight. Vitamin D supplements are available in a variety of forms, including tablets, capsules, and liquids. The recommended daily intake of vitamin D varies depending on age, sex, and other factors, and it is important to follow the dosage recommendations provided by a healthcare professional. There are several different forms of vitamin D available, with the two most popular being ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3).
See More Information Regarding Vitamin D| Ingredient Group | Vitamin D |
|---|---|
| Category | vitamin |
Vitamin E
Vitamin E is a fat-soluble vitamin found naturally in a variety of foods, including vegetable oils, nuts, seeds, and leafy green vegetables. Vitamin E is also available as a dietary supplement and is often added to skincare products. Vitamin E has many roles in the body, including protecting cells from oxidative stress and supporting immune function.
See More Information Regarding Vitamin E| Ingredient Group | Vitamin E |
|---|---|
| Category | vitamin |
Vitamin K
| Ingredient Group | Vitamin K |
|---|---|
| Category | vitamin |
Thiamine
| Ingredient Group | Thiamin |
|---|---|
| Category | vitamin |
- Vitamin B1
Riboflavin
| Ingredient Group | Riboflavin |
|---|---|
| Category | vitamin |
- Vitamin B2
Niacin
Niacin, also known as vitamin B3, is a water-soluble vitamin found in a variety of foods, including meat, poultry, fish, and fortified grains. It plays a variety of roles in the body, including in the metabolism of carbohydrates, fats, and proteins. It is also necessary for the production of energy in the body and in the synthesis of different hormones. Niacin deficiency is rare in developed countries, however, supplementation has been shown to have several positive benefits. For example, it can reduce the risk of diabetic neuropathy (i.e., nerve pain) and has been shown effective for treating some types of high cholesterol (extended-release niacin is used as a prescription drug in the United States for this purpose). There is a multitude of niacin forms available as dietary supplements, including NADH, niacinamide, and nicotinamide riboside, all with different properties.
See More Information Regarding Niacin| Ingredient Group | Niacin |
|---|---|
| Category | vitamin |
Vitamin B6
| Ingredient Group | Vitamin B6 |
|---|---|
| Category | vitamin |
Folic Acid
| Ingredient Group | Folate |
|---|---|
| Category | vitamin |
Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin crucial for several bodily functions. It plays a pivotal role in the formation of red blood cells, aiding in the prevention of anemia. Vitamin B12 is essential for maintaining a healthy nervous system and proper brain function, as it is involved in the synthesis of myelin, the protective sheath around nerve fibers. This vitamin is primarily found in animal-based foods such as meat, fish, dairy products, and eggs, making it important for vegetarians and vegans to consider supplementation. A deficiency in vitamin B12 can lead to neurological issues, fatigue, and cognitive impairment.
See More Information Regarding Vitamin B12| Ingredient Group | Vitamin B12 |
|---|---|
| Category | vitamin |
Biotin
Biotin, also known as vitamin H or vitamin B7, is a water-soluble vitamin that plays a role in various metabolic processes in the body. It is necessary for the growth and maintenance of healthy skin, hair, and nails, and is also involved in the metabolism of carbohydrates, fats, and amino acids. Biotin is considered to be generally safe when consumed in the recommended daily amounts but it is important to note that consuming large amounts of biotin-containing supplements or products can cause falsely high or falsely low test results for certain laboratory tests, such as those for thyroid function, hormone levels, and certain biomarkers for certain diseases.
See More Information Regarding Biotin| Ingredient Group | Biotin |
|---|---|
| Category | vitamin |
Pantothenic Acid
| Ingredient Group | Vitamin B5 |
|---|---|
| Category | vitamin |
Calcium
Calcium is a vital nutrient found in various foods such as dairy products, certain vegetables, and many fortified items. Over 99% of the body's calcium is stored in the bones and teeth, predominantly as hydroxyapatite. The remaining calcium circulates in the blood, extracellular fluid, muscles, and other tissues, where it is essential for processes like nerve signaling, muscle contraction, vascular activities, glandular secretion, and maintaining cell membrane and capillary permeability. It also plays critical roles in enzyme reactions, respiration, kidney function, and blood clotting, and is involved in neurotransmitter and hormone release, amino acid uptake, vitamin B12 absorption, and gastrin secretion. Calcium balance changes with age: it is positive during periods of growth, stable in adulthood, and tends to become negative in older age. Calcium loss occurs through feces, urine, sweat, and shedding skin cells. In women, reduced estrogen levels decrease calcium absorption and retention, increase bone turnover, and lead to lower bone mass. Calcium supplements come in various forms, including citrate and carbonate, which differ mainly in their calcium content and absorption rates. Calcium citrate is easily absorbed and can be taken without food, making it suitable for older adults or those with low stomach acid. In contrast, calcium carbonate, which contains a higher percentage of calcium, is best absorbed when taken with meals.
See More Information Regarding Calcium| Ingredient Group | Calcium |
|---|---|
| Category | mineral |
Iron
| Ingredient Group | Iron |
|---|---|
| Category | mineral |
Magnesium
Magnesium is a mineral that is essential for the proper functioning of the body. It plays a role in many important physiological processes, including the contraction and relaxation of muscles, the transmission of nerve impulses, and the regulation of blood pressure. Magnesium is found in a variety of foods, including leafy green vegetables, nuts, and grains, and it is also available as a dietary supplement. There are several different forms of magnesium that are available as supplements, all of which can be used to prevent deficiency. Additionally, magnesium is purported to have several different health benefits, such as improving sleep, reducing muscle cramps, reducing anxiety, and preventing or treating migraines. Although magnesium is essential for health, magnesium-containing foods and supplements can interact with some prescription medications if used at the same time.
See More Information Regarding Magnesium| Ingredient Group | Magnesium |
|---|---|
| Category | mineral |
Zinc
Zinc is a mineral that is essential for the proper functioning of the human body. It is involved in many important physiological processes, including immune system function, wound healing, taste, and smell. Zinc is found in a variety of foods, including meat, seafood, and whole grains, and it is also available as a dietary supplement. Zinc supplements may be used to treat or prevent zinc deficiency, which can occur due to certain medical conditions, such as gastrointestinal disorders, alcohol abuse, and certain medications. Zinc supplements may also be used for other purposes, such as to boost the immune system, improve acne, and reduce the severity and duration of colds. There are several different forms of zinc supplements available, including zinc gluconate, zinc acetate, and zinc sulfate. The most common form of zinc supplements is zinc gluconate, which is well absorbed and is less likely to cause stomach-related side effects than other forms of zinc.
See More Information Regarding Zinc| Ingredient Group | Zinc |
|---|---|
| Category | mineral |
Selenium
Selenium is an essential trace element that plays a crucial role in various physiological functions in the human body. It is often included in dietary supplements due to its antioxidant properties and its contribution to maintaining a healthy immune system. Selenium is important for thyroid function and may also have a role in protecting against certain chronic diseases. However, it's essential to note that excessive selenium intake can be harmful, so it's crucial to adhere to recommended daily allowances and consult with a healthcare professional before taking selenium supplements.
See More Information Regarding Selenium| Ingredient Group | Selenium |
|---|---|
| Category | mineral |
- L-Selenomethionine
Copper
| Ingredient Group | Copper |
|---|---|
| Category | mineral |
Manganese
Manganese is an essential trace mineral that plays a vital role in various physiological processes within the human body. It is required for proper brain function, bone formation, enzyme activation, and wound healing. Manganese also contributes to metabolism, antioxidant defense, and the formation of connective tissues.
See More Information Regarding Manganese| Ingredient Group | Manganese |
|---|---|
| Category | mineral |
Chromium
Chromium is a trace mineral that is found in small amounts in the human body. It is believed to play a role in the metabolism of carbohydrates, fats, and proteins. It is used as a dietary supplement for its purported ability to help with weight loss and blood sugar control. There is some evidence to suggest that chromium supplements may help to improve blood sugar control in people with type 2 diabetes, although the results of studies on this topic have been mixed. Some studies have found that chromium supplements can improve insulin sensitivity and reduce blood sugar levels in those with poorly controlled diabetes, while others have found no significant effects.
See More Information Regarding Chromium| Ingredient Group | Chromium |
|---|---|
| Category | mineral |
Drugs that interact with Women's Daily Multivitamin by up&up
Below is a list of drug interactions for each ingredient in this supplement product. Please note that a supplement product may contain more than one ingredient that has interactions.
Label Information
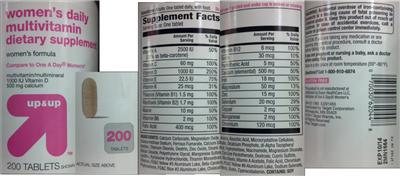
Supplement Facts:
| Daily Value (DV) Target Group(s): | Adults and children 4 or more years of age |
|---|---|
| Minimum serving Sizes: |
1 Tablet(s)
|
| Maximum serving Sizes: |
1 Tablet(s)
|
| UPC/BARCODE | 070030620448 |
| Ingredient | Amount per Serving | Group | % DV, Adults & children 4+ years |
|---|---|---|---|
| Vitamin A |
2500 IU
|
Vitamin A |
50%
|
| Vitamin C |
60 mg
|
Vitamin C |
100%
|
| Vitamin D |
1000 IU
|
Vitamin D |
250%
|
| Vitamin E |
22.5 IU
|
Vitamin E |
75%
|
| Vitamin K |
25 mcg
|
Vitamin K |
31%
|
| Thiamine |
1.5 mg
|
Thiamin |
100%
|
| Riboflavin |
1.7 mg
|
Riboflavin |
100%
|
| Niacin |
10 mg
|
Niacin |
50%
|
| Vitamin B6 |
2 mg
|
Vitamin B6 |
100%
|
| Folic Acid |
400 mcg
|
Folate |
100%
|
| Vitamin B12 |
6 mcg
|
Vitamin B12 |
100%
|
| Biotin |
30 mcg
|
Biotin |
10%
|
| Pantothenic Acid |
5 mg
|
Vitamin B5 |
50%
|
| Calcium |
500 mg
|
Calcium |
50%
|
| Iron |
18 mg
|
Iron |
100%
|
| Magnesium |
50 mg
|
Magnesium |
13%
|
| Zinc |
15 mg
|
Zinc |
100%
|
| Selenium |
20 mcg
|
Selenium |
29%
|
| Copper |
2 mg
|
Copper |
100%
|
| Manganese |
2 mg
|
Manganese |
100%
|
| Chromium |
120 mcg
|
Chromium |
100%
|
| Other Ingredients: |
Calcium Carbonate
Magnesium Oxide
Acacia
Ascorbic Acid
Microcrystalline Cellulose
Croscarmellose Sodium
Ferrous Fumarate
Calcium Silicate
Dicalcium Phosphate
DL-Alpha-Tocopheryl Acetate
Titanium Dioxide
Dextrin
Zinc Oxide
Stearic Acid
Hypromellose
Niacinamide
Magnesium Stearate
Silicon Dioxide
Polyethylene Glycol
Manganese Sulfate
Calcium Pantothenate
Dextrose
Cupric Sulfate
Lecithin
Pyridoxine Hydrochloride
Thiamine Mononitrate
Riboflavin
Vitamin A Acetate
Chromium Chloride
FD&C Yellow #5 Aluminum Lake
Beta-Carotene
FD&C Yellow #6 Aluminum Lake
Sodium Selenate
Phytonadione
FD&C Blue #2 Aluminum Lake
Cholecalciferol
Cyanocobalamin
|
|---|
Label Statments:
| FDA Statement of Identity |
- dietary supplement
|
|---|---|
| Formula |
- women's formula
- 1000 IU Vitamin D
500 mg calcium
- CONTAINS: SOY
|
| Brand IP Statement(s) |
- Compare to One A Day(R) Women's*
- This product is not manufactured or distributed by Bayer HealthCare LLC, distributor of One A Day(R) Women's.
- (C) 2011 Target Brands, Inc. All Rights Reserved
|
| General Statements |
- multivitamin/multimineral
- SHOWN ACTUAL SIZE ABOVE
- EXP10/14
2MN1664
- ID201455
|
| Storage |
- Store in a dry place at room temperature (59(0)-86(0)F).
|
| Suggested/Recommended/Usage/Directions |
- Directions: Adults: One tablet daily, with food.
|
| Precautions |
- Do not use if printed seal under cap is broken or missing
- WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
- CAUTION: If you are taking any medication or are planning any medical procedure, consult a doctor before taking this product. If you are pregnant or nursing a baby, ask a doctor before using this product.
|
| Formulation |
- GLUTEN FREE
|
| General |
- 094 02 1365 : 1F482 UW F2
|
Brand Information
See all products by this brand
| Distributed by | |
|---|---|
| Name | Target Corporation |
| City | Minneapolis |
| State | MN |
| ZipCode | 55403 |
| Shop | |
| Web Address | Target.com |
| Questions? Call | |
| Phone Number | 1-800-910-6874 |
Return to the main supplement interaction checker page
Parts of this content are provided by the Therapeutic Research Center, LLC and the Dietary Supplement Label Database.
DISCLAIMER: Currently this does not check for drug-drug interactions. This is not an all-inclusive comprehensive list of potential interactions and is for informational purposes only. Not all interactions are known or well-reported in the scientific literature, and new interactions are continually being reported. Input is needed from a qualified healthcare provider including a pharmacist before starting any therapy. Application of clinical judgment is necessary.
© 2021 Therapeutic Research Center, LLC
Drug descriptions are provided by MedlinePlus.