Search by Drug Name or NDC
NDC 00037-8130-56 MUSE 500 ug/1 Details
MUSE 500 ug/1
MUSE is a URETHRAL SUPPOSITORY in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Meda Pharmaceuticals Inc.. The primary component is ALPROSTADIL.
MedlinePlus Drug Summary
Alprostadil injection and suppositories are used to treat certain types of erectile dysfunction (impotence; inability to get or keep an erection) in men. Alprostadil injection is also sometimes used in combination with other tests to diagnose erectile dysfunction. Alprostadil is in a class of medications called vasodilators. It works by relaxing the muscles and blood vessels in the penis to keep enough blood in the penis so that an erection can occur. Alprostadil does not cure erectile dysfunction or increase sexual desire. Alprostadil does not prevent pregnancy or the spread of sexually transmitted diseases such as human immunodeficiency virus (HIV).
Related Packages: 00037-8130-56Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Alprostadil Urogenital
Product Information
| NDC | 00037-8130 |
|---|---|
| Product ID | 0037-8130_0209b68d-e74a-43a9-ad21-c9bca0293f0f |
| Associated GPIs | 40303010008930 |
| GCN Sequence Number | 029188 |
| GCN Sequence Number Description | alprostadil SUPP.URETH 500 MCG URETHRAL |
| HIC3 | F2A |
| HIC3 Description | DRUGS TO TREAT ERECTILE DYSFUNCTION (ED) |
| GCN | 02297 |
| HICL Sequence Number | 000177 |
| HICL Sequence Number Description | ALPROSTADIL |
| Brand/Generic | Brand |
| Proprietary Name | MUSE |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Alprostadil |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SUPPOSITORY |
| Route | URETHRAL |
| Active Ingredient Strength | 500 |
| Active Ingredient Units | ug/1 |
| Substance Name | ALPROSTADIL |
| Labeler Name | Meda Pharmaceuticals Inc. |
| Pharmaceutical Class | Genitourinary Arterial Vasodilation [PE], Prostaglandin Analog [EPC], Prostaglandin E1 Agonist [EPC], Prostaglandin Receptor Agonists [MoA], Prostaglandins [CS], Venous Vasodilation [PE] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA020700 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images



NDC 00037-8130-56 (00037813056)
| NDC Package Code | 0037-8130-56 |
|---|---|
| Billing NDC | 00037813056 |
| Package | 6 POUCH in 1 CARTON (0037-8130-56) / 1 SUPPOSITORY in 1 POUCH (0037-8130-01) |
| Marketing Start Date | 2011-07-22 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 4c55f3f9-c4cf-11df-851a-0800200c9a66 Details
DESCRIPTION
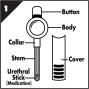
MUSE® (alprostadil) is a single-use, medicated transurethral system for the delivery of alprostadil to the male urethra. Alprostadil is suspended in polyethylene glycol 1450 (as excipient) and is formed into a medicated pellet (micro-suppository measuring 1.4 mm in diameter by 3 mm or 6 mm in length) that resides in the tip of a translucent hollow applicator. MUSE is administered by inserting the applicator stem into the urethra after urination. The pellet containing alprostadil is delivered by depressing the applicator button (see Figure 1). The components of the delivery system are constructed of medical grade polypropylene. Each MUSE system is packaged in an individual foil pouch.
The active ingredient in MUSE is alprostadil, which is chemically identical to the naturally occurring eicosanoid, prostaglandin E1 (PGE1). The chemical name for alprostadil is prost-13-en-1-oic acid, 11,15-dihydroxy-9-oxo-(11α,13E,15S)-(1R,2R,3R)-3-hydroxy-2-[(E)-(3S)-3-hydroxy-1-octenyl]-5-oxo-cyclopentane heptanoic acid, and the molecular weight is 354.49. The empirical formula is C20H34O5. The structural formula of alprostadil is represented below:
Alprostadil is a white to off-white crystalline powder with a melting point between 115° and 116°C. Its solubility at 35°C is 8000 mcg per 100 mL double-distilled water. The inactive ingredient in MUSE is polyethylene glycol 1450, USP. There are no other active agents or excipients in MUSE.
MUSE is available in 4 dosage strengths: 125 mcg, 250 mcg, 500 mcg, and 1000 mcg.
CLINICAL PHARMACOLOGY
Mechanism of Action
Prostaglandin E1 is a naturally occurring acidic lipid that is synthesized from fatty acid precursors by most mammalian tissues and has a variety of pharmacologic effects. Human seminal fluid is a rich source of prostaglandins, including PGE1 and PGE2, and the total concentration of prostaglandins in ejaculate has been estimated to be approximately 100-200 mcg/mL. In vitro, alprostadil (PGE1) has been shown to cause dose-dependent smooth muscle relaxation in isolated corpus cavernosum and corpus spongiosum preparations. Additionally, vasodilation has been demonstrated in isolated cavernosal artery segments that were pre-contracted with either norepinephrine or prostaglandin F2α. When alprostadil was injected into the corpus cavernosum of pigtail monkeys in vivo, dose-dependent increases in cavernosal artery blood flow were observed.
In human studies using Doppler duplex ultrasonography, intraurethral administration of 500 mcg of MUSE resulted in an increase in cavernosal artery diameter and a 5- to 10-fold increase in peak systolic flow velocities. These results suggest that intraurethral alprostadil is absorbed from the urethra, transported throughout the erectile bodies by communicating vessels between the corpus spongiosum and corpora cavernosa, and able to induce vasodilation of the targeted vascular beds.
The vasodilatory effects of alprostadil on the cavernosal arteries and the trabecular smooth muscle of the corpora cavernosa result in rapid arterial inflow and expansion of the lacunar spaces within the corpora. As the expanded corporal sinusoids are compressed against the tunica albuginea, venous outflow through subtunical vessels is impeded and penile rigidity develops. This process is referred to as the corporal veno-occlusive mechanism.
The most notable systemic effects of alprostadil are vasodilation, inhibition of platelet aggregation, and stimulation of intestinal and uterine smooth muscle. Intravenous doses of 1 to 10 micrograms per kilogram of body weight lower blood pressure in mammals by decreasing peripheral resistance. Reflex increases in cardiac output and heart rate may accompany these effects.
Pharmacokinetics
About 80% of alprostadil administered by MUSE is absorbed within 10 minutes and is rapidly cleared from the systemic circulation by the lungs, leaving barely detectable systemic blood levels.
Absorption
MUSE is designed to deliver alprostadil directly to the urethral lining for transfer via the corpus spongiosum to the corpora cavernosa. Intraurethral administration of MUSE is preceded by urination, and the residual urine disperses the medicated pellet, permitting alprostadil to be absorbed by the urethral mucosa. The transurethral absorption of alprostadil after MUSE administration is biphasic. Initial absorption is rapid, with approximately 80% of an administered dose absorbed within 10 minutes. The mean time to the maximum plasma PGE1 concentration after a 1000 mcg intraurethral dose of MUSE is approximately 16 minutes.
In 10 normal human volunteers, endogenous PGE1 levels in the ejaculate averaged 31 mcg (range 0-161 mcg). In these same volunteers, an average of 123 mcg of additional PGE1 (range 30-369 mcg) was present in the ejaculate obtained 10 minutes after the highest dose (1000 mcg) of MUSE. The mean total endogenous PGE content (PGE1, PGE2, 19-OH-PGE1, and 19-OH-PGE2) of the ejaculate in these subjects was 444 mcg (range 0-1423 mcg).
Distribution
Following MUSE administration, alprostadil is absorbed from the urethral mucosa into the corpus spongiosum. A portion of the administered dose is transported to the corpora cavernosa through collateral vessels, while the remainder passes into the pelvic venous circulation through veins draining the corpus spongiosum. The half-life of alprostadil in humans is short, varying between 30 seconds and 10 minutes, depending on the body compartment in which it is measured and the physiological status of the subject. Nearly all of the alprostadil entering the central venous circulation is removed in a single pass through the lungs; thus peripheral venous plasma levels of PGE1 are low or undetectable (< 2 picograms/mL) after MUSE administration. The mean maximum plasma PGE1 concentration following intraurethral administration of the highest dose of MUSE (1000 mcg) was barely detectable (11.4 picograms/mL). In a study of 14 subjects, the plasma PGE1 level was shown to be undetectable within 60 minutes of MUSE administration in most subjects.
Metabolism
Alprostadil is rapidly metabolized locally by enzymatic oxidation of the 15-hydroxyl group to 15-keto-PGE1. The enzyme catalyzing this process has been isolated from many tissues in the lower genitourinary tract including the urethra, prostate, and corpus cavernosum. 15-keto-PGE1 retains little (1-2%) of the biological activity of PGE1. 15-keto-PGE1 is rapidly reduced at the C13-C14 position to form the most abundant metabolite in plasma, 13,14-dihydro,15-keto PGE1 (DHK-PGE1), which is biologically inactive. The majority of DHK-PGE1 is further metabolized to smaller prostaglandin remnants that are cleared primarily by the kidney and liver. Between 60% and 90% of PGE1 has been shown to be metabolized after 1 pass through the pulmonary capillary beds.
Excretion
After intravenous administration of tritium-labeled alprostadil in man, labeled drug disappears rapidly from the blood in the first 10 minutes, and by 1 hour radioactivity in the blood reaches a low level. The metabolites of alprostadil are excreted primarily by the kidney, with approximately 90% of an administered intravenous dose excreted in the urine within 24 hours of dosing. The remainder is excreted in the feces. There is no evidence of tissue retention of alprostadil or its metabolites following intravenous administration.
Pharmacokinetics in Special Populations
Pulmonary Disease
The near-complete pulmonary first-pass metabolism of PGE1 is the primary factor influencing the systemic pharmacokinetics of MUSE and is a reason that peripheral venous plasma levels of PGE1 are low or undetectable (< 2 picograms/mL) following MUSE administration. Patients with pulmonary disease therefore may have a reduced capacity to clear the drug. In patients with the adult respiratory distress syndrome (ARDS), pulmonary extraction of intravascularly administered alprostadil was reduced by approximately 15% compared to a control group of patients with normal respiratory function (66 ± 3.2% vs. 78 ± 2.4%).
Geriatrics
The effects of age on the pharmacokinetics of alprostadil have not been evaluated.
CLINICAL TRIALS
The MUSE system was evaluated in 7 placebo-controlled trials of various design in over 2500 patients with a history of erectile dysfunction of various etiologies. These trials assessed erectile function in the clinic and sexual intercourse in outpatient settings. In studies of sexual performance, patients were screened in the clinic, generally using doses of 125 mcg to 1000 mcg, for a satisfactory erectile response, then sent home with the selected dose or placebo for evaluation of sexual performance. Not all patients beginning titration had a successful dose and some patients could not tolerate MUSE, principally because of penile pain, so that the success rates in the studies described below must be understood to represent response rates only in patients who were successfully titrated.
In 2 identical multicenter, double-blind, placebo-controlled, parallel-group studies, 1511 monogamous and heterosexual patients with a mean 4-year history of erectile dysfunction and at least a 3-month history of no erections adequate for sexual intercourse without medical assistance, were enrolled and began dose titration in the clinic with doses between 125 mcg and 1000 mcg. 996 patients (66%) completed dose titration, achieved an erection sufficient for intercourse, and were randomized equally to placebo or active treatment and followed during at-home treatment for up to 3 months. 874 patients and partners completed 3 months of follow-up. About 10%, 20%, 30%, and 40% of patients were titrated to 125 mcg, 250 mcg, 500 mcg, and 1000 mcg, respectively. Couples on active therapy were more likely to have at least 1 successful sexual intercourse (65% vs. 19%) than were couples on placebo. Among patients who reported successful intercourse at least once with active treatment, approximately 7 of 10 MUSE systems resulted in successful sexual intercourse. Results were similar in patients with erectile dysfunction stemming from surgery or trauma, diabetes, vascular disease, or other etiologies, and were similar in Caucasians and non-Caucasians. In administrations resulting in sexual intercourse, the duration of erections sufficient for penetration was 6 minutes on placebo and 16 minutes on active drug. Successful therapy with MUSE was associated with improvement in the quality of life measures of “emotional well-being” for patients and “relationship with partner” for both patients and their female partners.
INDICATIONS AND USAGE
CONTRAINDICATIONS
MUSE is contraindicated in men with any of the following:
- 1.
- Known hypersensitivity to alprostadil.
- 2.
- Abnormal penile anatomy: MUSE is contraindicated in patients with urethral stricture, balanitis (inflammation/infection of the glans of the penis), severe hypospadias and curvature, and in patients with acute or chronic urethritis.
- 3.
- Sickle cell anemia or trait, thrombocythemia, polycythemia, multiple myeloma: MUSE is contraindicated in patients who are prone to venous thrombosis or who have a hyperviscosity syndrome and are therefore at increased risk of priapism (rigid erection lasting 6 or more hours).
- 4.
- MUSE should not be used in men for whom sexual activity is inadvisable (see General Precautions).
- 5.
- MUSE should not be used for sexual intercourse with a pregnant woman unless the couple uses a condom barrier.
WARNINGS
Because of the potential for symptomatic hypotension and syncope, which occurred in 3% and 0.4%, respectively, of patients during in-clinic dosing, MUSE titration should be carried out under medical supervision. During post-marketing surveillance syncope occurring within one hour of administration has been reported. Patients should be cautioned to avoid activities, such as driving or hazardous tasks, where injury could result if hypotension or syncope were to occur after MUSE administration.
PRECAUTIONS
General Precautions
- 1.
- A complete medical history and physical examination should be undertaken to exclude reversible causes of erectile dysfunction prior to the initiation of MUSE therapy. In addition, underlying disorders that might preclude the use of MUSE (see CONTRAINDICATIONS) should be sought.
- 2.
- Cardiovascular effects: During in-clinic dosing, patients should be monitored for symptoms of hypotension, and the lowest effective dose of MUSE should be prescribed.
- 3.
- Hematologic effects: Patients administering MUSE improperly may be at risk of urethral abrasion resulting in minor bleeding or spotting. Patients on anticoagulant therapy or with bleeding disorders may be at higher risk of bleeding. Patients on anticoagulant therapy have been safely treated with MUSE; however, the risk/benefit ratio in these patients should be considered prior to prescribing MUSE.
- 4.
- Resumption of sexual activity: Sexual intercourse is considered a vigorous physical activity, and it increases heart rate as well as cardiac work. Physicians may want to examine the cardiac fitness of patients prior to treating erectile dysfunction.
- 5.
- Priapism and prolonged erection: In clinical trials of MUSE, priapism (rigid erection lasting ≥ 6 hours) and prolonged erection (rigid erection lasting 4 hours and < 6 hours) were reported infrequently (< 0.1% and 0.3% of patients, respectively). Nevertheless, these events are a potential risk of pharmacologic therapy and can cause penile injury. Physicians should lower the dose or consider discontinuing MUSE treatment in any patient who develops priapism or prolonged erection.
- 6.
- Drug-Drug Interactions: Because there are low or undetectable (< 2 picograms/mL) amounts of alprostadil found in the peripheral venous circulation following MUSE administration, systemic drug-drug interactions with MUSE are unlikely. Although formal studies have not been conducted, the concomitant use of MUSE and anti-hypertensive medications may increase the risk of hypotension. It is therefore advised that caution be used in the administration of MUSE to individuals on anti-hypertensive medications. In addition, the presence of medications in the circulation that attenuate erectile function may influence the response to MUSE.
- 7.
- Drug-Device Interactions: Use of MUSE in patients with penile implants has not been studied.
- 8.
- Sexual Preference: There is no experience in homosexual men and no experience with other than vaginal intercourse.
Information for Patients
Patients should be informed that MUSE offers no protection from the transmission of sexually transmitted diseases. Patients and partners who use MUSE need to be counseled about the protective measures that are necessary to guard against the spread of sexually transmitted agents, including the human immunodeficiency virus (HIV).
Although unreported in clinical trials, there is the possibility that an overdosage of MUSE can cause priapism, a painful erection of the penis sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious and, if untreated, it can lead to permanent inability to have an erection. Patients who experience a prolonged erection should seek prompt medical attention.
Patients should be instructed how to administer MUSE. A patient package insert must be given to each patient at the initiation of MUSE therapy.
Information for Partners
Partners of patients using MUSE should be informed that MUSE offers no protection from the transmission of sexually transmitted diseases. Patients and partners who use MUSE should be counseled about the protective measures that are necessary to guard against the spread of sexually transmitted agents, including the human immunodeficiency virus (HIV). Human semen contains PGE1, but additional amounts may be present from MUSE administration (see CLINICAL PHARMACOLOGY). Partners who have experienced an extended period of sexual abstinence should be encouraged to seek advice from a health care professional prior to resuming sexual intercourse. The use of a water-based lubricant may facilitate vaginal penetration.
It is recommended that couples using MUSE employ adequate contraception if the female partner is of childbearing potential. There is no information on the effects on early pregnancy of PGE1 at the levels received by female partners. MUSE has no contraceptive properties. MUSE should not be used if the female partner is pregnant, unless the couple uses a condom barrier.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies of alprostadil have not been conducted. Alprostadil showed no evidence of mutagenicity in vitro in the Ames bacterial reverse mutation test, the unscheduled DNA synthesis assay in rat hepatocytes, or the Chinese hamster ovary forward gene mutation assay; nor was there evidence of mutagenicity in vivo in the mouse micronucleus assay. Alprostadil concentrations increased chromosomal aberrations above control incidence in the in vitro Chinese hamster ovary chromosomal aberration assay.
In dogs, sperm concentration, morphology, and motility were unaffected by daily intraurethral administration of up to 3000 mcg MUSE (alprostadil) for 13 weeks (200 mcg/kg/day or about 3.5 times the maximum recommended daily dose adjusted for body surface area). Alprostadil concentrations of 400 mcg/mL had no effect on human sperm motility or viability in vitro.
Pregnancy
Alprostadil has been shown to be embryotoxic (decreased fetal weight) when administered as a subcutaneous bolus to pregnant rats at doses as low as500 mcg/kg/day. Doses of 2000 mcg/kg/day resulted in increased resorptions, reduced numbers of live fetuses, increased incidences of visceral and skeletal variations (primarily left umbilical artery and generalized reduction in ossification of the entire skeleton) and gross visceral and skeletal malformations (primarily edema, hydrocephaly, anophthalmia/microphthalmia, and skeletal anomalies). The latter dose produced maternal toxicity (ataxia, lethargy, diarrhea, and retarded body weight gain). When administered by continuous intravenous infusion, evidence of embryotoxicity (decreased fetal weight gain and increased incidence of hydroureter) was observed at 2000 mcg/kg/day, a dose that was also associated with a decrease in maternal weight gain. Intravaginal administration of up to 4000 mcg/day of MUSE (alprostadil) to pregnant rabbits (1100 mcg/kg/day or about 12.5 times the maximum recommended daily dose adjusted for body surface area) resulted in no evidence of harm to the fetus. MUSE should not be used for sexual intercourse with a pregnant woman unless the couple uses a condom barrier.
ADVERSE REACTIONS
In-Clinic Titration
In the 2 largest double-blind, parallel, placebo-controlled trials, 1511 patients received MUSE at least 1 time in the clinic setting. The most frequently reported drug-related side effects during in-clinic titration included pain in the penis (36%), urethra (13%), or testes (5%). These discomforts were most commonly reported as mild and transient, but about 7% of patients withdrew at this stage because of adverse events. Urethral bleeding/spotting and other minor abrasions to the urethra were reported in approximately 3% of patients. Symptomatic lowering of blood pressure (hypotension) occurred in 3% of patients; in addition, some lowering of blood pressure may occur without symptoms. Dizziness was reported in 4% of patients. Syncope (fainting) was reported by 0.4% of patients. (See WARNINGS).
Home Treatment
996 patients (66% of those who began titration) were studied during the home treatment portion of 2 Phase III placebo-controlled studies. Fewer than 2% of patients discontinued from these studies primarily because of adverse events. The following table summarizes the frequency of adverse events reported by patients using MUSE or placebo.
|
Event |
MUSE n = 486 |
Placebo n = 511 |
|
UROGENITAL SYSTEM | ||
|
Penile Pain |
32% |
3% |
|
Urethral Burning |
12% |
4% |
|
Minor Urethral Bleeding/Spotting |
5% |
1% |
|
Testicular Pain |
5% |
1% |
|
NERVOUS SYSTEM | ||
|
Dizziness |
2% |
< 1% |
|
BODY AS A WHOLE | ||
|
Flu Symptoms |
4% |
2% |
|
Headache |
3% |
2% |
|
Pain |
3% |
1% |
|
Accidental Injury |
3% |
2% |
|
Back Pain |
2% |
1% |
|
Pelvic Pain |
2% |
< 1% |
|
RESPIRATORY | ||
|
Rhinitis |
2% |
< 1% |
|
Infection |
3% |
2% |
Other drug-related side effects observed during in-clinic titration and home treatment include swelling of leg veins, leg pain, perineal pain, and rapid pulse, each occurring in < 2% of patients.
Female Partner Adverse Events
The most common drug-related adverse event reported by female partners during placebo-controlled clinical studies was vaginal burning/itching, reported by 5.8% of partners of patients on active vs. 0.8% of partners of patients on placebo. It is unknown whether this adverse event experienced by female partners was a result of the medication or a result of resuming sexual intercourse, which occurred much more frequently in partners of patients on active medication.
To report suspected adverse reactions, contact Meda Pharmaceuticals Inc. at 1-888-345-6873 or contact FDA at 1-800-FDA-1088, fax 1-800-FDA-0178 or online at www.fda.gov/medwatch/report.htm.
OVERDOSAGE
Overdosage has not been reported with MUSE. Overdosage with MUSE may result in hypotension, persistent penile pain and possibly priapism (rigid erection lasting ≥ 6h). Priapism can result in permanent worsening of erectile function. Patients suspected of overdosage who develop these symptoms should be kept under medical supervision until systemic or local symptoms have resolved.
DOSAGE AND ADMINISTRATION
MUSE is a transurethral delivery system available in 4 dosage strengths: 125 mcg, 250 mcg, 500 mcg, and 1000 mcg. MUSE should be administered as needed to achieve an erection. The onset of effect is within 5-10 minutes after administration. The duration of effect is approximately 30-60 minutes. However, the actual duration will vary from patient to patient. Each patient should be instructed by a medical professional on proper technique for administering MUSE prior to self-administration. The maximum frequency of use is no more than 2 systems per 24-hour period.
Initiation of Therapy
Dose titration should be administered under the supervision of a physician to test a patient's responsiveness to MUSE, to demonstrate proper administration technique (see detailed instructions for MUSE administration in patient package insert), and to monitor for evidence of hypotension (see WARNINGS). Patients should be individually titrated to the lowest dose that is sufficient for sexual intercourse. The lower doses of MUSE (125 mcg or 250 mcg) are recommended for initial dosing. If necessary, the dose should be increased (or decreased) on separate occasions in a stepwise manner until the patient achieves an erection that is sufficient for sexual intercourse.
HOW SUPPLIED
MUSE is supplied in individual foil pouches containing one (1) system per pouch. MUSE is available in unit cartons containing six (6) systems. MUSE is available in the following 4 dosage strengths:
|
Dosage |
NDC Numbers |
Identifying |
|
|
Strength |
Carton |
Pouch |
Package Color |
|
125 mcg |
0037-8110-06 |
0037-8110-01 |
Tan |
|
250 mcg |
0037-8120-06 |
0037-8120-01 |
Green |
|
500 mcg |
0037-8130-06 |
0037-8130-01 |
Blue |
|
1000 mcg |
0037-8140-06 |
0037-8140-01 |
Burgundy |
Rx Only.
STORAGE AND HANDLING
Store unopened foil pouches in a refrigerator at 2°-8°C (36°-46°F). Do not expose MUSE to temperatures above 30°C (86°F). MUSE may be kept by the patient at room temperature (below 30°C or 86°F) for up to 14 days prior to use.
Medical information line at Meda Pharmaceuticals Inc. 1-888-345-MUSE (1-888-345-6873).
MUSE is a registered trademark of Meda AB, a Mylan company.
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
http://www.muserx.net
Catalog Number: 05-10-00001F
IN-1001-04
Revised: 4/2018
©2018 Mylan Specialty L.P.
PATIENT INFORMATION
Please read this pamphlet before using MUSE® (alprostadil). This pamphlet is a quick reference source on important information about MUSE for you and your partner. Before administering MUSE, please review the patient video and education booklet. These materials provide visual instruction and more detailed information as well as practical tips on how to use MUSE.
WHAT IS MUSE?
MUSE represents a unique approach for the treatment of erectile dysfunction, commonly called impotence. It is based on the discovery that the urethra (the normal pathway for urine) can absorb certain medications into the surrounding erectile tissues thereby creating an erection. There are 4 dose strengths available: 125, 250, 500, and 1000 micrograms. The MUSE applicator (Fig.1) contained in each foil pouch is intended for 1 administration only. Your dose of MUSE will be determined by you and your physician. After administration, the erection process will begin within 5 – 10 minutes, and may last 30 – 60 minutes. However, the actual duration will vary from patient to patient.
WHAT IS MUSE USED FOR?
MUSE is indicated for the treatment of erectile dysfunction. Erectile dysfunction is the inability to attain or maintain an erection sufficient for sexual intercourse.
WHO SHOULD NOT USE MUSE?
You should not use MUSE if you have any of the following:
- •
- Known hypersensitivity to alprostadil (the active medication in MUSE)
- •
- An abnormally formed penis
- •
- Have been advised not to undertake sexual activity
- •
- Conditions that might result in long-lasting erections, such as sickle cell anemia or trait, leukemia, or tumor of the bone marrow (multiple myeloma)
- •
- MUSE should not be used for sexual intercourse with a pregnant woman unless the couple uses a condom barrier.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF MUSE?
The most common side effects that have been observed using MUSE follow:
- •
- Aching in the penis, testicles, legs, and in the perineum (area between the penis and rectum)
- •
- Warmth or burning sensation in the urethra
- •
- Redness of the penis due to increased blood flow
- •
- Minor urethral bleeding or spotting due to improper administration.
Side effects reported less frequently:
- •
- Prolonged erection - PLEASE NOTE: IF YOUR ERECTION IS RIGID FOR MORE THAN 4 HOURS, CALL YOUR DOCTOR PROMPTLY.
- •
- Swelling of leg veins
- •
- Light-headedness/Dizziness
- •
- Fainting - PLEASE NOTE: AFTER USING MUSE, YOU SHOULD AVOID ACTIVITIES, SUCH AS DRIVING OR HAZARDOUS TASKS, WHERE INJURY COULD RESULT IF DIZZINESS OR FAINTING WERE TO OCCUR. IN PATIENTS EXPERIENCING THESE SYMPTOMS, THE SYMPTOMS HAVE USUALLY OCCURRED DURING INITIATION OF THERAPY AND WITHIN ONE HOUR OF MUSE ADMINISTRATION.
- •
- Rapid pulse.
If you have a history of fainting be sure to discuss this with your doctor prior to using MUSE. If you do experience dizziness or feel faint, this may be due to the lowering of your blood pressure. Lie down immediately and raise your legs. If symptoms persist, call your doctor promptly. Because of the potential for these side effects, MUSE titration should be carried out under medical supervision.
Call your doctor for medical advice about side effects. To report side effects, contact Meda Pharmaceuticals Inc. at 1-888-367-6873 or contact FDA at 1-800-FDA-1088, fax 1-800-FDA-0178 or online at www.fda.gov/medwatch/report.htm.
Changing Your Dosage
It is assumed that you and your doctor have determined the proper dose of MUSE. If you suspect that your dose needs to be increased or decreased to achieve the response that works best for you, please call your doctor to determine if your dose needs to be reevaluated. Do not use MUSE more than twice in a 24-hour period.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF MUSE FOR YOUR PARTNER?
The most common reported side effects observed in women whose partners use MUSE are mild vaginal itching or burning. Using a water-based lubricant can help to make vaginal penetration easier. Your partner may want to consult her health care provider if she has not had sexual intercourse for an extended period of time.
IMPORTANT INFORMATION FOR YOU AND YOUR PARTNER
Pregnancy
MUSE has no contraceptive properties.
Because MUSE has not been tested during human pregnancy, it is recommended that couples use adequate contraception if the female partner is of childbearing potential. MUSE should not be used for sexual intercourse with a pregnant woman unless the couple uses a condom barrier.
Sexually Transmitted Diseases
MUSE will not protect you or your partner from sexually transmitted diseases like chlamydia, gonorrhea, herpes simplex virus, viral hepatitis, human immunodeficiency virus (HIV - the virus that causes AIDS), human papilloma virus (genital warts), and syphilis. Latex condoms can protect against these sexually transmitted diseases.
HOW SHOULD I STORE MUSE?
It is recommended that MUSE be stored in a refrigerator. MUSE may be kept at room temperature (less than 30°C/86°F) for up to 14 days prior to use. It is very important that MUSE not be exposed to temperatures above 30°C/86°F since this will make MUSE ineffective. MUSE should not be exposed to high temperatures or placed in direct sunlight.
Storage When Traveling
When traveling, store MUSE in a portable ice pack or cooler. Do not store in the trunk of a car or in baggage storage areas where MUSE may be exposed to extremes in temperature.
HOW TO ADMINISTER MUSE:
- 1.
- Immediately prior to administration, urinate and gently shake the penis several times to remove excess urine. A moist urethra makes administration of MUSE easier. The medicated pellet has been specially developed to dissolve in the small quantity of urine that remains in the urethra after urination.
- 2.
- Open the foil pouch by tearing fully across from the notched edge (Fig. 2). Let the MUSE slide out of the pouch. Save the pouch for discarding the MUSE applicator later.
- 1.
- To remove the protective cover from the applicator stem (Fig. 3), hold the body of the applicator with your thumb and forefinger. Twist the body and pull out the applicator from the cover, being careful not to push in or pull out the applicator button. Avoid touching the applicator stem and tip. Save the cover for discarding the MUSE applicator later.
- 1.
- Visually inspect the MUSE. The MUSE system is see-through, and you will be able to see the medicated pellet at the end of the stem. Make sure that the pellet is present before insertion (Fig. 4).
- 1.
- Hold the applicator in a way which is the most comfortable for you. (Fig. 5a and 5b)
- 1.
- Please review Figure 6a, the anatomy of the penis.
- While sitting or standing, whichever is more comfortable for you, take several seconds to gently and slowly stretch the penis upward to its full length, with gentle compression from top to bottom of the glans (Fig. 6b). This straightens and opens the urethra. Slowly insert the MUSE stem into the urethra up to the collar (Fig. 6c). If you feel any discomfort or a pulling sensation, withdraw the applicator slightly and then gently reinsert.
- 1.
- Gently and completely push down (Fig. 7) the button at the top of the applicator until it stops. It is important to do this to ensure that the medicated pellet is completely released. Hold the applicator in this position for 5 seconds.
- 1.
- Gently rock the applicator from side to side. This will separate the medicated pellet from the applicator tip (Fig. 8). If you apply too much pressure you may scratch the lining of the urethra causing it to bleed.
- 1.
- Remove the applicator while keeping the penis upright.
- 2.
- Visually inspect the applicator tip to see that the medication is no longer in the applicator. Do not touch the stem. If you notice some residual medication in the end of the applicator, gently reinsert into the urethra and repeat steps 7, 8, and 9.
- 3.
- Holding the penis upright and stretched to its full length, roll the penis firmly between your hands for at least 10 seconds. This will ensure that the medication is adequately distributed along the walls of the urethra (Fig. 9). If you feel a burning sensation, it may help to continue to roll the penis for an additional 30 – 60 seconds or until the burning subsides.
- 1.
- Remember, each MUSE is good for a single administration only. Replace the cover on the MUSE applicator, place in the opened foil pouch, fold, and discard as normal household waste.
After you have administered MUSE, it is important to sit, or preferably stand or walk about for 10 minutes while the erection is developing. This increases blood flow to the penis and will enhance your erection.
ADDITIONAL INFORMATION AND PRACTICAL TIPS
Factors Which May Enhance Your Erection:
- •
- Being well rested and relaxed
- •
- Sexual foreplay with your partner or self-stimulation while sitting or standing
- •
- Pelvic exercises (for example, Kegel exercises) - these consist of tightening and releasing your pelvic and buttock muscles. These are the muscles you use to stop urination
- •
- Various positions that may favor blood flow into the penis. Please refer to the patient starter booklet and video for illustrative examples.
Factors Which May Reduce Your Erection:
- •
- Anxiety, fatigue, tension, and too much alcohol
- •
- Lying on your back too soon after administration of MUSE may decrease blood flow to the penis and result in loss of erection
- •
- Urination or dribbling immediately following administration may result in loss of medication from the urethra
- •
- Using medications that contain decongestants, such as over-the-counter cold remedies, allergy, sinus medications, and appetite suppressants, may block the effect of MUSE.
COMMONLY ASKED QUESTIONS ABOUT MUSE
Will insertion of MUSE hurt?
At first, you may feel some minor discomfort from insertion. Urinating prior to administration will reduce the chance of discomfort or abrasions and is important for dissolving the medicated pellet. Be sure to straighten your penis to its full length when inserting the MUSE applicator. With repeated use, administration will become much easier.
What are the side effects associated with MUSE?
Most of the side effects reported in men are relatively minor and include burning and aching in the penis and groin. Rarely noted are prolonged erection, light-headedness, dizziness, fainting, rapid pulse, and swelling of the leg veins. If you feel dizzy, light-headed, faint, or experience rapid pulse, lie down immediately and raise your legs. If symptoms persist, call your doctor promptly. Because of the potential for these side effects, MUSE titration should be carried out under medical supervision.
(See also: “WHAT ARE THE POSSIBLE SIDE EFFECTS OF MUSE?” on the other side.)
In women, mild vaginal itching and burning have been observed.
After I administer MUSE, can we immediately lie down and begin sexual activity?
You can begin sexual activity, but having the man lie down, especially on his back shortly after administration, is not recommended. This will reduce blood flow to the penis and may reduce the erection. It is important to sit, stand or walk about for 10 minutes after administration. Many couples have used this time to incorporate various types of foreplay. After this initial period, you can assume different positions leading to sexual intercourse. Some couples have noticed that the erection is better maintained in positions that favor blood flow into the penis during intercourse.
Please review the video and patient starter booklet available from your doctor which illustrates various positions that will enhance your erection.
How long will the effect of MUSE last?
An erection should begin within 5 – 10 minutes after administering MUSE. The duration of effect is approximately 30 – 60 minutes. However, the actual duration will vary from patient to patient.
What will the erection be like? How will it compare to the erections I had when I was younger?
An effective dose of MUSE should produce an erection sufficient for sexual intercourse. MUSE may not create an erection such as those you experienced when you were younger. Some patients may experience some mild pain and aching in the penis or groin area. Also, your erection may continue after orgasm.
How do I know if I have the correct dose of MUSE?
You and your physician will determine the appropriate dose of MUSE. If your erection cannot be maintained for the time needed to have foreplay and sexual intercourse, you may need to have your dose increased. Similarly, an erection that lasts longer than desired may require a dose decrease. Call your doctor if you suspect you may require a dosing adjustment.
After my erection is over, will my penis feel sensitive?
Your penis may feel full, warm, and somewhat sensitive to the touch. These effects are normal and may last a few hours.
Can I reuse MUSE?
No. MUSE is intended for single-dose application only.
How do I dispose of the MUSE applicator?
After you have administered MUSE, replace the cover on the applicator, place in the opened foil pouch, fold, and discard as normal household waste.
If my erection lasts longer than desired, what should I do?
Note: Call your doctor promptly if you have a rigid erection that lasts more than 4 hours.
An application of ice packs to the inner thigh may shorten the duration of the erection, since the cold will restrict blood flow to the penis. If used, ice packs should be applied alternately to each inner thigh for a period not exceeding 10 minutes.
How often can I safely use MUSE?
MUSE should not be used more than twice per day.
If you have any additional questions about MUSE, please call the toll free patient information line at Meda Pharmaceuticals Inc. 1-888-367-MUSE (1-888-367-6873), or visit the MUSE product web site, http://www.muserx.net
MUSE is a registered trademark of Meda AB, a Mylan company.
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
http://www.muserx.net
Catalog Number: 05-10-00000F
IS-1001-04
Revised: 4/2018
©2018 Mylan Specialty L.P.
PRINCIPAL DISPLAY PANEL – 125 mcg
NDC 0037-8110-06
MUSE®
(alprostadil)
urethral suppository
Transurethral System 125 mcg
Store in a refrigerator at 2°C to 8°C (36°F to 46°F).
Please read patient instruction leaflet regarding usage and storage.
Rx only
Contents: 6 single-use systems
MUSE and MEDA PHARMACEUTICALS are registered trademarks, and the
MEDA PHARMACEUTICALS logo is a trademark of Meda AB or a related entity.
U.S. Patent No. 5,886,039
http://www.muserx.net
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
CAT. NO. 06-10-01256E
UC-100100-04
Revised 06/2017
PRINCIPAL DISPLAY PANEL – 250 mcg
NDC 0037-8120-06
MUSE®
(alprostadil)
urethral suppository
Transurethral System 250 mcg
Store in a refrigerator at 2°C to 8°C (36°F to 46°F).
Please read patient instruction leaflet regarding usage and storage.
Rx only
Contents: 6 single-use systems
MUSE and MEDA PHARMACEUTICALS are registered trademarks, and the
MEDA PHARMACEUTICALS logo is a trademark of Meda AB or a related entity.
U.S. Patent No. 5,886,039
http://www.muserx.net
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
CAT. NO. 06-10-02506E
UC-100200-04
Revised 06/2017
PRINCIPAL DISPLAY PANEL – 500 mcg
NDC 0037-8130-06
MUSE®
(alprostadil)
urethral suppository
Transurethral System 500 mcg
Store in a refrigerator at 2°C to 8°C (36°F to 46°F).
Please read patient instruction leaflet regarding usage and storage.
Rx only
Contents: 6 single-use systems
MUSE and MEDA PHARMACEUTICALS are registered trademarks, and the
MEDA PHARMACEUTICALS logo is a trademark of Meda AB or a related entity.
U.S. Patent No. 5,886,039
http://www.muserx.net
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
CAT. NO. 06-10-05006E
UC-100300-04
Revised 06/2017
PRINCIPAL DISPLAY PANEL – 1000 mcg
NDC 0037-8140-06
MUSE®
(alprostadil)
urethral suppository
Transurethral System 1000 mcg
Store in a refrigerator at 2°C to 8°C (36°F to 46°F).
Please read patient instruction leaflet regarding usage and storage.
Rx only
Contents: 6 single-use systems
MUSE and MEDA PHARMACEUTICALS are registered trademarks, and the
MEDA PHARMACEUTICALS logo is a trademark of Meda AB or a related entity.
U.S. Patent No. 5,886,039
http://www.muserx.net
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
CAT. NO. 06-10-10006E
UC-100400-05
Revised 2/2018
INGREDIENTS AND APPEARANCE
| MUSE
alprostadil suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| MUSE
alprostadil suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| MUSE
alprostadil suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| MUSE
alprostadil suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Meda Pharmaceuticals Inc. (051229602) |