Search by Drug Name or NDC
NDC 00093-5150-01 Moexipril Hydrochloride 15 mg/1 Details
Moexipril Hydrochloride 15 mg/1
Moexipril Hydrochloride is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Teva Pharmaceuticals USA, Inc.. The primary component is MOEXIPRIL HYDROCHLORIDE.
MedlinePlus Drug Summary
Moexipril is used to treat high blood pressure. Moexipril is in a class of medications called angiotensin-converting enzyme (ACE) inhibitors. It decreases certain chemicals that tighten the blood vessels, so blood flows more smoothly and the heart can pump blood more efficiently. High blood pressure is a common condition and when not treated, can cause damage to the brain, heart, blood vessels, kidneys, and other parts of the body. Damage to these organs may cause heart disease, a heart attack, heart failure, stroke, kidney failure, loss of vision, and other problems. In addition to taking medication, making lifestyle changes will also help to control your blood pressure. These changes include eating a diet that is low in fat and salt, maintaining a healthy weight, exercising at least 30 minutes most days, not smoking, and using alcohol in moderation.
Related Packages: 00093-5150-01Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Moexipril
Product Information
| NDC | 00093-5150 |
|---|---|
| Product ID | 0093-5150_47bb5b1f-0ee0-469f-9fcc-fc3087b2e865 |
| Associated GPIs | 36100033100320 |
| GCN Sequence Number | 023592 |
| GCN Sequence Number Description | moexipril HCl TABLET 15 MG ORAL |
| HIC3 | A4D |
| HIC3 Description | ANTIHYPERTENSIVES, ACE INHIBITORS |
| GCN | 48562 |
| HICL Sequence Number | 009934 |
| HICL Sequence Number Description | MOEXIPRIL HCL |
| Brand/Generic | Generic |
| Proprietary Name | Moexipril Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Moexipril Hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 15 |
| Active Ingredient Units | mg/1 |
| Substance Name | MOEXIPRIL HYDROCHLORIDE |
| Labeler Name | Teva Pharmaceuticals USA, Inc. |
| Pharmaceutical Class | Angiotensin Converting Enzyme Inhibitor [EPC], Angiotensin-converting Enzyme Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA076204 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 00093-5150-01 (00093515001)
| NDC Package Code | 0093-5150-01 |
|---|---|
| Billing NDC | 00093515001 |
| Package | 100 TABLET, FILM COATED in 1 BOTTLE (0093-5150-01) |
| Marketing Start Date | 2003-05-08 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.8792 |
| Pricing Unit | EA |
| Effective Date | 2023-12-20 |
| NDC Description | MOEXIPRIL HCL 15 MG TABLET |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 4 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 5d4078f3-b621-475f-a4e6-005de2e62b32 Details
WARNING
DESCRIPTION
Moexipril hydrochloride, USP, the hydrochloride salt of moexipril, is chemically described as [3S-[2[R*(R*)],3R*]]-2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic acid, monohydrochloride. It is a non-sulfhydryl containing precursor of the active angiotensin-converting enzyme (ACE) inhibitor moexiprilat and its structural formula is:
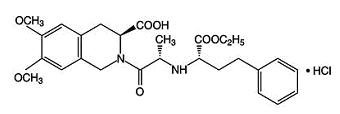
C27H34N2O7•HCl M.W. 535.04
Moexipril hydrochloride, USP is a fine white to off-white powder. It is soluble (about 10% weight-to-volume) in distilled water at room temperature.
Moexipril hydrochloride tablets USP are supplied as bisected, coated tablets containing 7.5 mg and 15 mg of moexipril hydrochloride, USP for oral administration. In addition to the active ingredient, moexipril hydrochloride, USP, the tablet core contains the following inactive ingredients: crospovidone, lactose monohydrate, magnesium stearate, pregelatinized starch and sodium bicarbonate. The film coating of the 7.5 mg tablet contains: hypromellose, iron oxide red, lactose monohydrate, titanium dioxide and triacetin. The film coating of the 15 mg tablet contains: hypromellose, iron oxide red, lactose monohydrate, titanium dioxide and triacetin.
Moexipril hydrochloride tablets USP meet USP Dissolution Test 2.
CLINICAL PHARMACOLOGY
Mechanism of Action
Moexipril hydrochloride is a prodrug for moexiprilat, which inhibits ACE in humans and animals. The mechanism through which moexiprilat lowers blood pressure is believed to be primarily inhibition of ACE activity. ACE is a peptidyl dipeptidase that catalyzes the conversion of the inactive decapeptide angiotensin I to the vasoconstrictor substance angiotensin II. Angiotensin II is a potent peripheral vasoconstrictor that also stimulates aldosterone secretion by the adrenal cortex and provides negative feedback on renin secretion. ACE is identical to kininase II, an enzyme that degrades bradykinin, an endothelium-dependent vasodilator. Moexiprilat is about 1000 times as potent as moexipril in inhibiting ACE and kininase II. Inhibition of ACE results in decreased angiotensin II formation, leading to decreased vasoconstriction, increased plasma renin activity, and decreased aldosterone secretion. The latter results in diuresis and natriuresis and a small increase in serum potassium concentration (mean increases of about 0.25 mEq/L were seen when moexipril was used alone, see PRECAUTIONS).
Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of moexipril remains to be elucidated. Although the principal mechanism of moexipril in blood pressure reduction is believed to be through the renin-angiotensin-aldosterone system, ACE inhibitors have some effect on blood pressure even in apparent low-renin hypertension. As is the case with other ACE inhibitors, however, the antihypertensive effect of moexipril is considerably smaller in black patients, a predominantly low-renin population, than in non-black hypertensive patients.
Pharmacokinetics and Metabolism
Pharmacokinetics
Moexipril’s antihypertensive activity is almost entirely due to its deesterified metabolite, moexiprilat. Bioavailability of oral moexipril is about 13% compared to intravenous (I.V.) moexipril (both measuring the metabolite moexiprilat), and is markedly affected by food, which reduces the peak plasma level (Cmax) and AUC (see Absorption). Moexipril should therefore be taken in a fasting state. The time of peak plasma concentration (Tmax) of moexiprilat is about 1½ hours and elimination half-life (t1/2) is estimated at 2 to 9 hours in various studies, the variability reflecting a complex elimination pattern that is not simply exponential. Like all ACE inhibitors, moexiprilat has a prolonged terminal elimination phase, presumably reflecting slow release of drug bound to the ACE. Accumulation of moexiprilat with repeated dosing is minimal, about 30%, compatible with a functional elimination t1/2 of about 12 hours. Over the dose range of 7.5 to 30 mg, pharmacokinetics are approximately dose proportional.
Absorption
Moexipril is incompletely absorbed, with bioavailability as moexiprilat of about 13%. Bioavailability varies with formulation and food intake which reduces Cmax and AUC by about 70% and 40% respectively after the ingestion of a low-fat breakfast or by 80% and 50% respectively after the ingestion of a high-fat breakfast.
Distribution
The clearance (CL) for moexipril is 441 mL/min and for moexiprilat 232 mL/min with a t1/2 of 1.3 and 9.8 hours, respectively. Moexiprilat is about 50% protein bound. The volume of distribution of moexiprilat is about 183 liters.
Metabolism and Excretion
Moexipril is relatively rapidly converted to its active metabolite moexiprilat, but persists longer than some other ACE inhibitor prodrugs, such that its t1/2 is over one hour and it has a significant AUC. Both moexipril and moexiprilat are converted to diketopiperazine derivatives and unidentified metabolites. After I.V. administration of moexipril, about 40% of the dose appears in urine as moexiprilat, about 26% as moexipril, with small amounts of the metabolites; about 20% of the I.V. dose appears in feces, principally as moexiprilat. After oral administration, only about 7% of the dose appears in urine as moexiprilat, about 1% as moexipril, with about 5% as other metabolites. Fifty-two percent of the dose is recovered in feces as moexiprilat and 1% as moexipril.
Special Populations
Decreased renal function
The effective elimination of t1/2 and AUC of both moexipril and moexiprilat are increased with decreasing renal function. There is insufficient information available to characterize this relationship fully, but at creatinine clearances in the range of 10 to 40 mL/min, the t1/2 of moexiprilat is increased by a factor of 3 to 4.
Pharmacodynamics and Clinical Effect
Single and multiple doses of 15 mg or more of moexipril hydrochloride tablets gives sustained inhibition of plasma ACE activity of 80 to 90%, beginning within 2 hours and lasting 24 hours (80%).
In controlled trials, the peak effects of orally administered moexipril increased with the dose administered over a dose range of 7.5 to 60 mg, given once a day. Antihypertensive effects were first detectable about 1 hour after dosing, with a peak effect between 3 and 6 hours after dosing. Just before dosing (i.e., at trough), the antihypertensive effects were less prominently related to dose and the antihypertensive effect tended to diminish during the 24 hour dosing interval when the drug was administered once a day.
In multiple dose studies in the dose range of 7.5 to 30 mg once daily, moexipril hydrochloride tablets lowered sitting diastolic and systolic blood pressure effects at trough by 3 to 6 mmHg and 4 to 11 mmHg more than placebo, respectively. There was a tendency toward increased response with higher doses over this range. These effects are typical of ACE inhibitors but, to date, there are no trials of adequate size comparing moexipril with other antihypertensive agents.
The trough diastolic blood pressure effects of moexipril were approximately 3 to 6 mmHg in various studies. Generally, higher doses of moexipril leave a greater fraction of the peak blood pressure effect still present at trough. During dose titration, any decision as to the adequacy of a dosing regimen should be based on trough blood pressure measurements. If diastolic blood pressure control is not adequate at the end of the dosing interval, the dose can be increased or given as a divided (BID) regimen.
During chronic therapy, the antihypertensive effect of any dose of moexipril hydrochloride is generally evident within 2 weeks of treatment, with maximal reduction after 4 weeks. The antihypertensive effects of moexipril hydrochloride have been proven to continue during therapy for up to 24 months.
Moexipril hydrochloride, like other ACE inhibitors, is less effective in decreasing trough blood pressures in blacks than in non-blacks. Placebo-corrected trough group mean diastolic blood pressure effects in blacks in the proposed dose range varied between +1 to -3 mmHg compared with responses in non-blacks of -4 to -6 mmHg.
The effectiveness of moexipril hydrochloride was not significantly influenced by patient age, gender, or weight. Moexipril hydrochloride has been shown to have antihypertensive activity in both pre- and postmenopausal women who have participated in placebo-controlled clinical trials.
Formal interaction studies with moexipril have not been carried out with antihypertensive agents other than thiazide diuretics. In these studies, the added effect of moexipril was similar to its effect as monotherapy. In general, ACE inhibitors have less than additive effects with beta-adrenergic blockers, presumably because both work by inhibiting the renin-angiotensin system.
INDICATIONS AND USAGE
Moexipril hydrochloride tablets USP are indicated for treatment of patients with hypertension. It may be used alone or in combination with thiazide diuretics.
In using moexipril hydrochloride tablets USP, consideration should be given to the fact that another ACE inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen-vascular disease. Available data are insufficient to show that moexipril hydrochloride tablets USP do not have a similar risk (see WARNINGS).
In considering use of moexipril hydrochloride tablets USP, it should be noted that in controlled trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks. In addition, ACE inhibitors (for which adequate data are available) cause a higher rate of angioedema in black than in non-black patients (see WARNINGS,Angioedema).
CONTRAINDICATIONS
Moexipril hydrochloride tablets are contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an ACE inhibitor.
Do not coadminister aliskiren with moexipril hydrochloride in patients with diabetes (see PRECAUTIONS, Drug Interactions).
WARNINGS
Anaphylactoid and Possibly Related Reactions
Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors, including moexipril hydrochloride, may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema
Angioedema involving the face, extremities, lips, tongue, glottis, and/or larynx has been reported in patients treated with ACE inhibitors, including moexipril hydrochloride. Symptoms suggestive of angioedema or facial edema occurred in < 0.5% of moexipril-treated patients in placebo-controlled trials. None of the cases were considered life-threatening and all resolved either without treatment or with medication (antihistamines or glucocorticoids). One patient treated with hydrochlorothiazide alone experienced laryngeal edema. No instances of angioedema were reported in placebo-treated patients.
In cases of angioedema, treatment should be promptly discontinued and the patient carefully observed until the swelling disappears. In instances where swelling has been confined to the face and lips, the condition has generally resolved without treatment, although antihistamines have been useful in relieving symptoms.
Angioedema associated with involvement of the tongue, glottis, or larynx, may be fatal due to airway obstruction. Appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 to 0.5 mL) and/or measures to ensure a patent airway, should be promptly provided (see ADVERSE REACTIONS).
Intestinal Angioedema
Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid Reactions During Desensitization
Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions did not occur when ACE inhibitors were temporarily withheld, but they reappeared when the ACE inhibitors were inadvertently readministered.
Anaphylactoid Reactions During Membrane Exposure
Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hypotension
Moexipril hydrochloride can cause symptomatic hypotension, although, as with other ACE inhibitors, this is unusual in uncomplicated hypertensive patients treated with moexipril hydrochloride alone. Symptomatic hypotension was seen in 0.5% of patients given moexipril and led to discontinuation of therapy in about 0.25%. Symptomatic hypotension is most likely to occur in patients who have been salt- and volume-depleted as a result of prolonged diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Volume- and salt-depletion should be corrected and, in general, diuretics stopped, before initiating therapy with moexipril hydrochloride (see PRECAUTIONS, Drug Interactions, and ADVERSE REACTIONS).
In patients with congestive heart failure, with or without associated renal insufficiency, ACE inhibitor therapy may cause excessive hypotension, which may be associated with oliguria or progressive azotemia, and rarely, with acute renal failure and death. In these patients, moexipril hydrochloride therapy should be started under close medical supervision, and patients should be followed closely for the first two weeks of treatment and whenever the dose of moexipril or an accompanying diuretic is increased. Care in avoiding hypotension should also be taken in patients with ischemic heart disease, aortic stenosis, or cerebrovascular disease, in whom an excessive decrease in blood pressure could result in a myocardial infarction or a cerebrovascular accident.
If hypotension occurs, the patient should be placed in a supine position and, if necessary, treated with an intravenous infusion of normal saline. Moexipril hydrochloride treatment usually can be continued following restoration of blood pressure and volume.
Neutropenia/Agranulocytosis
Another ACE inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression, rarely in patients with uncomplicated hypertension, but more frequently in hypertensive patients with renal impairment, especially if they also have a collagen-vascular disease such as systemic lupus erythematosus or scleroderma. Although there were no instances of severe neutropenia (absolute neutrophil count < 500/mm3) among patients given moexipril hydrochloride, as with other ACE inhibitors, monitoring of white blood cell counts should be considered for patients who have collagen-vascular disease, especially if the disease is associated with impaired renal function. Available data from clinical trials of moexipril hydrochloride are insufficient to show that moexipril hydrochloride does not cause agranulocytosis at rates similar to captopril.
Fetal Toxicity
Pregnancy category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue moexipril hydrochloride as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue moexipril hydrochloride, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to moexipril hydrochloride for hypotension, oliguria, and hyperkalemia (see PRECAUTIONS, Pediatric Use).
No embryotoxic, fetotoxic, or teratogenic effects were seen in rats or in rabbits treated with up to 90.9 and 0.7 times, respectively, the Maximum Recommended Human Dose (MRHD) on a mg/m2 basis.
Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and sometimes death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
PRECAUTIONS
General
Impaired Renal Function
As a consequence of inhibition of the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. There is no clinical experience of moexipril hydrochloride in the treatment of hypertension in patients with renal failure.
Some hypertensive patients with no apparent preexisting renal vascular disease have developed increases in blood urea nitrogen and serum creatinine, usually minor and transient, especially when moexipril hydrochloride has been given concomitantly with a thiazide diuretic. This is more likely to occur in patients with preexisting renal impairment. There may be a need for dose adjustment of moexipril hydrochloride and/or the discontinuation of the thiazide diuretic.
Evaluation of hypertensive patients should always include assessment of renal function (see DOSAGE AND ADMINISTRATION).
Hypertensive Patients With Congestive Heart Failure
In hypertensive patients with severe congestive heart failure, whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with ACE inhibitors, including moexipril hydrochloride, may be associated with oliguria and/or progressive azotemia and, rarely, acute renal failure and/or death.
Hypertensive Patients With Renal Artery Stenosis
In hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine have been observed in some patients following ACE inhibitor therapy. These increases were almost always reversible upon discontinuation of the ACE inhibitor and/or diuretic therapy. In such patients, renal function should be monitored during the first few weeks of therapy.
Hyperkalemia
In clinical trials, persistent hyperkalemia (serum potassium above 5.4 mEq/L) occurred in approximately 1.3% of hypertensive patients receiving moexipril hydrochloride. Risk factors for the development of hyperkalemia with ACE inhibitors include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements, and/or potassium-containing salt substitutes, which should be used cautiously, if at all, with moexipril hydrochloride (see PRECAUTIONS, Drug Interactions).
Surgery/Anesthesia
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, moexipril may block the effects of compensatory renin release. If hypotension occurs in this setting and is considered to be due to this mechanism, it can be corrected by volume expansion.
Cough
Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough. In controlled trials with moexipril, cough was present in 6.1% of moexipril patients and 2.2% of patients given placebo.
Information for Patients
Food
Patients should be advised to take moexipril one hour before meals (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Angioedema
Angioedema, including laryngeal edema, may occur with treatment with ACE inhibitors, usually occurring early in therapy (within the first month). Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of the face, extremities, eyes, lips, tongue, difficulty in breathing) and to take no more moexipril hydrochloride until they have consulted with the prescribing physician.
Symptomatic Hypotension
Patients should be cautioned that lightheadedness can occur with moexipril hydrochloride, especially during the first few days of therapy. If fainting occurs, the patient should stop taking moexipril hydrochloride and consult the prescribing physician.
All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; patients should be advised to consult their physician if they develop these conditions.
Hyperkalemia
Patients should be told not to use potassium supplements or salt substitutes containing potassium without consulting their physician.
Drug Interactions
Diuretics
Excessive reductions in blood pressure may occur in patients on diuretic therapy when ACE inhibitors are started. The possibility of hypotensive effects with moexipril hydrochloride can be minimized by discontinuing diuretic therapy for several days or cautiously increasing salt intake before initiation of treatment with moexipril hydrochloride. If this is not possible, the starting dose of moexipril should be reduced (see WARNINGS and DOSAGE AND ADMINISTRATION).
Potassium Supplements and Potassium-Sparing Diuretics
Moexipril hydrochloride can increase serum potassium because it decreases aldosterone secretion. Use of potassium-sparing diuretics (spironolactone, triamterene, amiloride) or potassium supplements concomitantly with ACE inhibitors can increase the risk of hyperkalemia. Therefore, if concomitant use of such agents is indicated, they should be given with caution and the patient’s serum potassium should be monitored.
Oral Anticoagulants
Interaction studies with warfarin failed to identify any clinically important effect on the serum concentrations of the anticoagulant or on its anticoagulant effect.
Lithium
Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium. These drugs should be coadministered with caution, and frequent monitoring of serum lithium levels is recommended. If a diuretic is also used, the risk of lithium toxicity may be increased.
Gold
Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including moexipril hydrochloride.
Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDS, including selective COX-2 inhibitors, with ACE inhibitors, including moexipril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving moexipril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including moexipril, may be attenuated by NSAIDS.
Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function and electrolytes in patients on moexipril hydrochloride and other agents that affect the RAS.
Do not coadminister aliskiren with moexipril hydrochloride in patients with diabetes. Avoid use of aliskiren with moexipril hydrochloride in patients with renal impairment (GFR < 60 mL/min).
Other Agents
No clinically important pharmacokinetic interactions occurred when moexipril hydrochloride was administered concomitantly with hydrochlorothiazide, digoxin, or cimetidine.
Moexipril hydrochloride has been used in clinical trials concomitantly with calcium-channel-blocking agents, diuretics, H2 blockers, digoxin, oral hypoglycemic agents, and cholesterol-lowering agents. There was no evidence of clinically important adverse interactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenicity was detected in long-term studies in mice and rats at doses up to 14 or 27.3 times the Maximum Recommended Human Dose (MRHD) on a mg/m2 basis.
No mutagenicity was detected in the Ames test and microbial reverse mutation assay, with and without metabolic activation, or in an in vivo nucleus anomaly test. However, increased chromosomal aberration frequency in Chinese hamster ovary cells was detected under metabolic activation conditions at a 20 hour harvest time.
Reproduction studies have been performed in rabbits at oral doses up to 0.7 times the MRHD on a mg/m2 basis, and in rats up to 90.9 times the MRHD on a mg/m2 basis. No indication of impaired fertility, reproductive toxicity, or teratogenicity was observed.
Nursing Mothers
It is not known whether moexipril hydrochloride is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when moexipril hydrochloride is given to a nursing mother.
Pediatric Use
Neonates with a history of in utero exposure to moexipril hydrochloride:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
Safety and effectiveness of moexipril hydrochloride in pediatric patients have not been established.
Geriatric Use
Clinical studies of moexipril hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Moexipril hydrochloride has been evaluated for safety in more than 2500 patients with hypertension; more than 250 of these patients were treated for approximately one year. The overall incidence of reported adverse events was only slightly greater in patients treated with moexipril hydrochloride than patients treated with placebo.
Reported adverse experiences were usually mild and transient, and there were no differences in adverse reaction rates related to gender, race, age, duration of therapy, or total daily dosage within the range of 3.75 mg to 60 mg. Discontinuation of therapy because of adverse experiences was required in 3.4% of patients treated with moexipril hydrochloride and in 1.8% of patients treated with placebo. The most common reasons for discontinuation in patients treated with moexipril hydrochloride were cough (0.7%) and dizziness (0.4%).
All adverse experiences considered at least possibly related to treatment that occurred at any dose in placebo-controlled trials of once-daily dosing in more than 1% of patients treated with moexipril hydrochloride alone and that were at least as frequent in the moexipril hydrochloride group as in the placebo group are shown in the following table:
|
ADVERSE EVENT |
MOEXIPRIL HYDROCHLORIDE (N = 674) |
PLACEBO (N = 226) |
|
N (%) |
N (%) |
|
|
Cough Increased |
41 (6.1) |
5 (2.2) |
|
Dizziness |
29 (4.3) |
5 (2.2) |
|
Diarrhea |
21 (3.1) |
5 (2.2) |
|
Flu Syndrome |
21 (3.1) |
0 (0) |
|
Fatigue |
16 (2.4) |
4 (1.8) |
|
Pharyngitis |
12 (1.8) |
2 (0.9) |
|
Flushing |
11 (1.6) |
0 (0) |
|
Rash |
11 (1.6) |
2 (0.9) |
|
Myalgia |
9 (1.3) |
0 (0) |
Other adverse events occurring in more than 1% of patients on moexipril that were at least as frequent on placebo include: headache, upper respiratory infection, pain, rhinitis, dyspepsia, nausea, peripheral edema, sinusitis, chest pain, and urinary frequency. See WARNINGS and PRECAUTIONS for discussion of anaphylactoid reactions, angioedema, hypotension, neutropenia/agranulocytosis, second and third trimester fetal/neonatal morbidity and mortality, hyperkalemia, and cough.
Other potentially important adverse experiences reported in controlled or uncontrolled clinical trials in less than 1% of moexipril patients or that have been attributed to other ACE inhibitors include the following:
Cardiovascular
Symptomatic hypotension, postural hypotension, or syncope were seen in 9/1750 (0.51%) patients; these reactions led to discontinuation of therapy in controlled trials in 3/1254 (0.24%) patients who had received moexipril hydrochloride monotherapy and in 1/344 (0.3%) patients who had received moexipril hydrochloride with hydrochlorothiazide (see PRECAUTIONS and WARNINGS). Other adverse events included angina/myocardial infarction, palpitations, rhythm disturbances, and cerebrovascular accident.
Renal
Of hypertensive patients with no apparent preexisting renal disease, 1% of patients receiving moexipril hydrochloride alone and 2% of patients receiving moexipril hydrochloride with hydrochlorothiazide experienced increases in serum creatinine to at least 140% of their baseline values (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Gastrointestinal
Abdominal pain, constipation, vomiting, appetite/weight change, dry mouth, pancreatitis, hepatitis.
Dermatologic
Apparent hypersensitivity reactions manifested by urticaria, rash, pemphigus, pruritus, photosensitivity, alopecia.
Other
Angioedema (see WARNINGS), taste disturbances, tinnitus, sweating, malaise, arthralgia, hemolytic anemia.
Clinical Laboratory Test Findings
Creatinine and Blood Urea Nitrogen
As with other ACE inhibitors, minor increases in blood urea nitrogen or serum creatinine, reversible upon discontinuation of therapy, were observed in approximately 1% of patients with essential hypertension who were treated with moexipril hydrochloride. Increases are more likely to occur in patients receiving concomitant diuretics and in patients with compromised renal function (see PRECAUTIONS, General).
Other (Causal Relationship Unknown)
Clinically important changes in standard laboratory tests were rarely associated with moexipril hydrochloride administration.
Elevations of liver enzymes and uric acid have been reported. In trials, less than 1% of moexipril-treated patients discontinued moexipril hydrochloride treatment because of laboratory abnormalities. The incidence of abnormal laboratory values with moexipril was similar to that in the placebo-treated group.
OVERDOSAGE
Human overdoses of moexipril have not been reported. In case reports of overdoses with other ACE inhibitors, hypotension has been the principal adverse effect noted. Single oral doses of 2 g/kg moexipril were associated with significant lethality in mice. Rats, however, tolerated single oral doses of up to 3 g/kg.
No data are available to suggest that physiological maneuvers (e.g., maneuvers to change the pH of the urine) would accelerate elimination of moexipril and its metabolites. The dialyzability of moexipril is not known.
Angiotensin II could presumably serve as a specific antagonist-antidote in the setting of moexipril overdose, but angiotensin II is essentially unavailable outside of research facilities. Because the hypotensive effect of moexipril is achieved through vasodilation and effective hypovolemia, it is reasonable to treat moexipril overdose by infusion of normal saline solution. In addition, renal function and serum potassium should be monitored.
DOSAGE AND ADMINISTRATION
Hypertension
The recommended initial dose of moexipril hydrochloride tablets in patients not receiving diuretics is 7.5 mg, one hour prior to meals, once daily. Dosage should be adjusted according to blood pressure response. The antihypertensive effect of moexipril hydrochloride tablets may diminish towards the end of the dosing interval. Blood pressure should, therefore, be measured just prior to dosing to determine whether satisfactory blood pressure control is obtained. If control is not adequate, increased dose or divided dosing can be tried. The recommended dose range is 7.5 to 30 mg daily, administered in one or two divided doses one hour before meals. Total daily doses above 60 mg a day have not been studied in hypertensive patients.
In patients who are currently being treated with a diuretic, symptomatic hypotension may occasionally occur following the initial dose of moexipril hydrochloride tablets. The diuretic should, if possible, be discontinued for 2 to 3 days before therapy with moexipril hydrochloride tablets is begun, to reduce the likelihood of hypotension (see WARNINGS). If the patient’s blood pressure is not controlled with moexipril hydrochloride tablets alone, diuretic therapy may then be reinstituted. If diuretic therapy cannot be discontinued, an initial dose of 3.75 mg of moexipril hydrochloride tablets should be used with medical supervision until blood pressure has stabilized (see WARNINGS and PRECAUTIONS, Drug Interactions).
HOW SUPPLIED
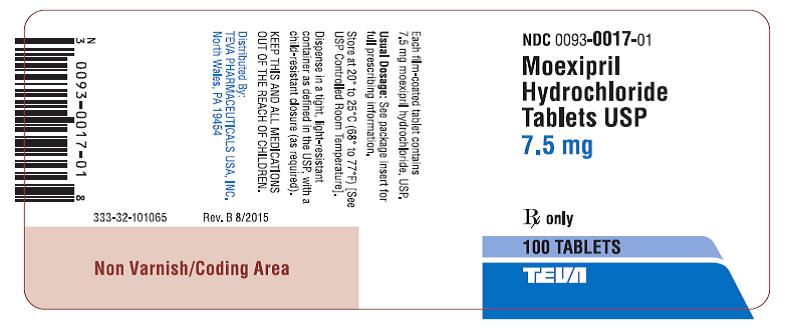
Moexipril hydrochloride tablets USP, 7.5 mg, are pink, film-coated, oval, convex tablets debossed “17” on one side and bisected on the other. They are available in bottles of 100. (NDC 0093-0017-01)
Moexipril hydrochloride tablets USP, 15 mg, are pink, film-coated, oval, convex tablets debossed “5150” on one side and “93” to the left of a bisect on the other side. They are available in bottles of 100. (NDC 0093-5150-01)
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Distributed By:
TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454
Rev. G 8/2015
Package/Label Display Panel
Package/Label Display Panel
INGREDIENTS AND APPEARANCE
| MOEXIPRIL HYDROCHLORIDE
moexipril hydrochloride tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| MOEXIPRIL HYDROCHLORIDE
moexipril hydrochloride tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Teva Pharmaceuticals USA, Inc. (001627975) |