Search by Drug Name or NDC
NDC 00113-0285-08 Good Sense 24 hour allergy nasal 50 ug/1 Details
Good Sense 24 hour allergy nasal 50 ug/1
Good Sense 24 hour allergy nasal is a NASAL SPRAY, METERED in the HUMAN OTC DRUG category. It is labeled and distributed by L. Perrigo Company. The primary component is FLUTICASONE PROPIONATE.
MedlinePlus Drug Summary
Nonprescription fluticasone nasal spray (Flonase Allergy) is used to relieve symptoms of rhinitis such as sneezing and a runny, stuffy, or itchy nose and itchy, watery eyes caused by hay fever or other allergies (caused by an allergy to pollen, mold, dust, or pets). Prescription fluticasone is also used to relieve symptoms of nonallergic rhinitis such as sneezing and runny or stuffy nose which are not caused by allergies. Prescription fluticasone nasal spray (Xhance) is used to treat nasal polyps (swelling of the lining of the nose). Fluticasone nasal spray should not be used to treat symptoms (e.g., sneezing, stuffy, runny, itchy nose) caused by the common cold. Fluticasone is in a class of medications called corticosteroids. It works by blocking the release of certain natural substances that cause allergy symptoms.
Related Packages: 00113-0285-08Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Fluticasone Nasal Spray
Product Information
| NDC | 00113-0285 |
|---|---|
| Product ID | 0113-0285_7c10d357-5137-42f1-8abb-bd05ce9f9b9e |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Good Sense 24 hour allergy nasal |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Fluticasone Propionate |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | SPRAY, METERED |
| Route | NASAL |
| Active Ingredient Strength | 50 |
| Active Ingredient Units | ug/1 |
| Substance Name | FLUTICASONE PROPIONATE |
| Labeler Name | L. Perrigo Company |
| Pharmaceutical Class | Corticosteroid Hormone Receptor Agonists [MoA], Corticosteroid [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA207957 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 00113-0285-08 (00113028508)
| NDC Package Code | 0113-0285-08 |
|---|---|
| Billing NDC | 00113028508 |
| Package | 1 BOTTLE in 1 CARTON (0113-0285-08) / 72 SPRAY, METERED in 1 BOTTLE |
| Marketing Start Date | 2021-09-02 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 6f3f64e6-d1f8-40d7-bb25-f6f279db9987 Details
Uses
Do not use
Ask a doctor or pharmacist before use if you are taking
When using this product
Stop use and ask a doctor if
- •
- you have, or come into contact with someone who has, chicken pox, measles or tuberculosis
- •
- your symptoms do not get better within 7 days of starting use or you get new symptoms such as severe facial pain or thick nasal discharge. You may have something more than allergies, such as an infection.
- •
- you get a constant whistling sound from your nose. This may be a sign of damage inside your nose.
- •
- you get an allergic reaction to this product. Seek medical help right away.
- •
- you get new changes to your vision that develop after starting this product
- •
- you have severe or frequent nosebleeds
Keep out of reach of children.
Directions
- •
- read the Quick Start Guide for how to:
- •
- prime the bottle
- •
- use the spray
- •
- clean the spray nozzle
- •
- shake gently before each use
- •
- use this product only once a day
- •
- do not use more than directed
|
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER |
|
|
CHILDREN 4 TO 11 YEARS OF AGE |
|
|
CHILDREN UNDER 4 YEARS OF AGE |
|
Inactive ingredients
Consumer Information
FLUTICASONE PROPIONATE NASAL SPRAY QUESTIONS & ANSWERS
WHAT FLUTICASONE PROPIONATE NASAL SPRAY IS, HOW TO USE IT, AND WHAT TO EXPECT.
UNDERSTANDING FLUTICASONE PROPIONATE NASAL SPRAY
What Fluticasone Propionate Nasal Spray is, and how it works.
What is Fluticasone Propionate Nasal Spray?
Fluticasone Propionate Nasal Spray is an effective, allergy medicine that you can now buy without a prescription. It works directly in the nose to help block your allergic reactions. Fluticasone Propionate Nasal Spray relieves allergy symptoms caused by pollen, mold, dust and pets.
What is the active ingredient in Fluticasone Propionate Nasal Spray?
The active ingredient in Fluticasone Propionate Nasal Spray is called fluticasone propionate. Fluticasone propionate is a glucocorticoid – a substance produced naturally by your body to help fight inflammation. It works in your nose to relieve your allergy symptoms. Barely any of it travels through your body. It’s been prescribed by doctors for over 20 years, and used effectively by millions.
A glucocorticoid is a kind of steroid that is different from the anabolic (muscle-building) steroids sometimes misused by athletes. In fact, world class athletes are allowed to use glucocorticoids during competition to treat their allergies.
Who should not use Fluticasone Propionate Nasal Spray?
For complete guidance, check the Drug Facts label on the back of the Fluticasone Propionate Nasal Spray package.
Can I use Fluticasone Propionate Nasal Spray for asthma?
No, Fluticasone Propionate Nasal Spray is not a treatment for asthma. Ask your doctor what medicine to take for your asthma.
Can I use Fluticasone Propionate Nasal Spray for colds?
No, use Fluticasone Propionate Nasal Spray to treat only your allergies – not your cold symptoms. If you’re not sure whether your symptoms come from allergies or a cold, ask your doctor.
What happens when you have allergies?
Allergies start when allergens like pollen or pet dander enter your body and trigger your immune system to respond.
Your body’s natural response is to release multiple inflammatory substances, (also referred to as mediators) that cause your allergy symptoms.
How does Fluticasone Propionate Nasal Spray work?
Fluticasone Propionate Nasal Spray works right in your nose to help block your allergic reaction at the source to relieve the symptoms that make you uncomfortable.
Fluticasone Propionate Nasal Spray acts on multiple inflammatory substances, including histamine, prostaglandins, cytokines, tryptases, chemokines and leukotrienes.
Most common OTC allergy pills act on histamine alone.
Because of the way it works, it may take several days for Fluticasone Propionate Nasal Spray to reach maximum effect. That’s why it’s best to use Fluticasone Propionate Nasal Spray regularly, once a day.
Fluticasone Propionate Nasal Spray not only relieves sneezing, itchy nose, runny nose and itchy, watery eyes, but also relieves nasal congestion.
Who should not use Fluticasone Propionate Nasal Spray or check with a doctor first?
Some people should not use Fluticasone Propionate Nasal Spray, or need to check with a health professional first. This table offers a quick summary of these situations.
|
If you… |
Here’s what to do… |
|
Are younger than 4 |
Do not use Fluticasone Propionate Nasal Spray |
|
Are pregnant or breast-feeding |
Talk to a health professional before using Fluticasone Propionate Nasal Spray |
|
Have or had glaucoma or cataracts |
Talk to your doctor before using Fluticasone Propionate Nasal Spray |
|
Have an injury or surgery to your nose that is not fully healed |
Do not use Fluticasone Propionate Nasal Spray |
|
Have ever had an allergic reaction to Fluticasone Propionate Nasal Spray or any of its ingredients |
Do not use Fluticasone Propionate Nasal Spray |
|
Are taking a medicine for HIV infection (such as ritonavir) |
Talk to your doctor or pharmacist before using Fluticasone Propionate Nasal Spray |
|
Are taking ketoconazole pills (medicine for fungal infection) |
Talk to your doctor or pharmacist before using Fluticasone Propionate Nasal Spray |
|
Are using a steroid medicine for asthma, allergies, skin rash, allergic reactions, inflammation or eye conditions |
Talk to your doctor or pharmacist before using Fluticasone Propionate Nasal Spray |
What problems can Fluticasone Propionate Nasal Spray help with?
Allergies can cause uncomfortable symptoms like congestion and itchy eyes. These symptoms can be triggered by allergens like pollen, mold, dust or pet dander.
Fluticasone Propionate Nasal Spray helps relieve a broad range of symptoms from many allergens.
For example, Fluticasone Propionate Nasal Spray helps with:
USING FLUTICASONE PROPIONATE NASAL SPRAY
How to get the best results with Fluticasone Propionate Nasal Spray.
Read the Drug Facts label on the back of the package or the Quick Start Guide on the other side for simple directions on using Fluticasone Propionate Nasal Spray.
Watch for purple color - it shows where there is different information for children ages 4-11, compared to users age 12 or older.
If you still have questions about using Fluticasone Propionate Nasal Spray after reading the Quick Start Guide, read this section for answers.
I know how to use a nasal spray. Why do I have to follow the directions?
If you don’t use the spray bottle correctly, you might not get a full dose. Without a full dose each time, you might not get the relief you deserve.
Follow the simple directions in the Quick Start Guide on other side.
If my symptoms go away, should I stop using Fluticasone Propionate Nasal Spray?
You may be tempted to stop using Fluticasone Propionate Nasal Spray when you start to feel better. It’s important you keep using Fluticasone Propionate Nasal Spray daily as long as you’re exposed to allergens that bother you, like pollen, mold, dust or pet dander. This way you’ll keep feeling relief.
If you suffer allergy symptoms only during certain times, like when pollen levels are high, you may stop using Fluticasone Propionate Nasal Spray when that time ends. If you are age 12 or older and need to use daily for longer than 6 months or age 4-11 and need to use for longer than 2 months a year, check with your doctor.
What if I miss a dose by accident?
If you miss a dose, just use your regular dose the next day. Don’t add an extra dose to make up for it.
Can I keep using Fluticasone Propionate Nasal Spray year round?
Some people suffer from allergies all year. If you are age 12 or older and have used Fluticasone Propionate Nasal Spray for six months or age 4-11 and have used for two months a year, check with your doctor to make sure it’s OK to keep using Fluticasone Propionate Nasal Spray. In fact, it’s a good idea for anyone with persistent allergies to talk with a doctor every so often about symptoms and medicines.
Can I share my Fluticasone Propionate Nasal Spray?
Do not share a bottle of Fluticasone Propionate Nasal Spray. Sharing the bottle can spread germs, because you insert the nozzle in your nose.
Can I spray Fluticasone Propionate Nasal Spray in my eyes or mouth?
No, Fluticasone Propionate Nasal Spray is meant to work only in your nose to relieve your allergy symptoms, including itchy, watery eyes.
Never spray Fluticasone Propionate Nasal Spray in your eyes or your mouth.
Some decongestants may increase blood pressure. Does Fluticasone Propionate Nasal Spray increase blood pressure?
When used as directed, Fluticasone Propionate Nasal Spray does not increase blood pressure.
Won’t I waste product by priming?
It’s not a waste to prime the pump, because it helps you get a full dose. Getting a full dose is important for getting the relief you deserve. See the Quick Start Guide for when and how to prime the pump.
Don’t worry about running out due to priming. There is enough medicine in the spray bottle to allow for priming sprays plus the number of sprays labeled on the bottle.
Always point the spray bottle away from your face when priming.
How long should a bottle of Fluticasone Propionate Nasal Spray last?
This table shows roughly how long your bottle will last. It assumes you follow the instructions for priming the pump, and that those age 12 or older use two sprays in each nostril every day while children age 4-11 use one spray in each nostril every day.
After you’ve used the number of sprays shown on the label, each spray may not deliver a full dose — even if there is liquid left in the bottle.
|
If the label says… |
The bottle should last age 12+… |
The bottle should last age 4-11… |
|
60 sprays |
2 weeks |
4 weeks |
|
72 sprays |
2 weeks |
5 weeks |
|
120 sprays |
4 weeks |
8 weeks |
|
144 sprays |
5 weeks |
10 weeks* *Check with a doctor if a child 4-11 needs to use longer than 2 months a year |
Is Fluticasone Propionate Nasal Spray OK to use with other medicines?
You should tell your doctor about all the medicines you take, including Fluticasone Propionate Nasal Spray. Fluticasone Propionate Nasal Spray can be used with most non-prescription and prescription medicines. However, there are a few medicines to look out for because they may cause the level of Fluticasone Propionate Nasal Spray in your body to become too high.
Just to be safe, check this table to see if you’re taking any of these medicines.
|
If you’re taking… |
Here’s what to do… |
|
Medicines for HIV infection (such as ritonavir) |
Talk to your doctor or pharmacist before using Fluticasone Propionate Nasal Spray |
|
Medicines with glucocorticoids Including some medicines for skin rash such as eczema, asthma, inflammation, allergic reactions, or eye conditions |
Talk to your doctor or pharmacist before using Fluticasone Propionate Nasal Spray |
|
Ketoconazole Pills for fungal infection |
Talk to your doctor or pharmacist before using Fluticasone Propionate Nasal Spray |
What are the differences in the way children age 4-11 should use Fluticasone Propionate Nasal Spray?
Children age 4-11 should use a lower dose of Fluticasone Propionate Nasal Spray for a shorter period of time.
|
Ages |
Children 4-11 years of age |
Users 12 years of age and older |
|
Dosage |
1 spray in each nostril once daily |
Up to 2 sprays in each nostril once daily |
|
Duration before checking with a doctor |
Up to 2 months of use a year |
Up to 6 months of daily use |
Why is the use of Fluticasone Propionate Nasal Spray for children age 4-11 limited to 2 months a year before checking with a doctor?
When used long-term, intranasal glucocorticoids like Fluticasone Propionate Nasal Spray may cause the growth rate of some children to be slower. Whether this will affect a child’s ultimate height is not known. As a precaution, children should use for the shortest amount of time necessary to achieve symptom relief. Talk to your child’s doctor if your child needs to use the spray for longer than 2 months a year.
WHAT TO EXPECT
What it’s like to use Fluticasone Propionate Nasal Spray.
How soon will I get relief?
You may start to feel relief the first day you use Fluticasone Propionate Nasal Spray. Keep using it every day, though. It takes several days before Fluticasone Propionate Nasal Spray builds up to full effectiveness.
How long will the relief last?
Fluticasone Propionate Nasal Spray is meant to control your symptoms every day, all day and all night. To help you get this lasting relief, it’s important to use Fluticasone Propionate Nasal Spray regularly, once a day.
Does Fluticasone Propionate Nasal Spray cause a “rebound” effect?
No, Fluticasone Propionate Nasal Spray does not cause a rebound effect.
Some nasal decongestant sprays may cause your nasal passages to swell up even more when you use them too often or for longer than their label says you should (three days). This is sometimes called a “rebound effect.”
Fluticasone Propionate Nasal Spray is a different kind of medicine and does not cause any rebound effect. You can use Fluticasone Propionate Nasal Spray for up to six months if you are age 12 or older or up to two months a year if you are age 4-11 before checking with a doctor.
Will Fluticasone Propionate Nasal Spray make me drowsy?
No, Fluticasone Propionate Nasal Spray does not cause drowsiness. Some allergy medications can cause drowsiness, but Fluticasone Propionate Nasal Spray does not.
Why does Fluticasone Propionate Nasal Spray have a smell?
The light floral scent you may notice comes from one of the important ingredients in the Fluticasone Propionate Nasal Spray formula. No fragrance is added to Fluticasone Propionate Nasal Spray.
What if little or nothing is spraying out?
Try priming the spray bottle. It may take a few pumps to get the dispenser spraying again.
If that doesn’t work, the spray nozzle may be clogged. You can clean it following the directions in the Quick Start Guide on the other side.
What if I feel stinging in my nose, or I sneeze?
Some people may feel a slight stinging, or may sneeze, after spraying Fluticasone Propionate Nasal Spray in their nostrils.
This feeling should go away in a few seconds.
What if I feel or taste the medicine in my throat?
Generally, you can avoid this by taking a shallower breath next time you spray Fluticasone Propionate Nasal Spray into your nostrils. For example, take the kind of breath you would use to smell a flower.
What if I have chicken pox, measles or tuberculosis or come into contact with someone who does?
Stop using Fluticasone Propionate Nasal Spray and ask your doctor.
What if I’m having severe sinus pain?
If you feel severe pain in your face, have thick nasal discharge, or think you may have a sinus infection, stop using Fluticasone Propionate Nasal Spray and see your doctor. Your doctor may want to consider if other medicines are needed.
What if my symptoms aren’t better after one week?
If you have used Fluticasone Propionate Nasal Spray for a week and your allergy symptoms are not getting better, stop use and ask your doctor. You may have an infection.
Does Fluticasone Propionate Nasal Spray have side effects?
Serious side effects are rare with Fluticasone Propionate Nasal Spray because Fluticasone Propionate Nasal Spray works in your nose, and barely any of it travels through your body. However, like all medicines, Fluticasone Propionate Nasal Spray can cause side effects in some people.
Here are some side effects that have been reported when using Fluticasone Propionate Nasal Spray. If you have any concerns about side effects, talk with your doctor.
|
Side effect |
What could happen |
What to do |
|
Allergic reaction to the product |
|
If you feel any of these symptoms, stop using Fluticasone Propionate Nasal Spray and see a doctor right away. |
|
Nose injury |
|
Apply pressure to your nose. Stop using Fluticasone Propionate Nasal Spray and see a doctor. |
|
This could be a sign of damage to your nose. Stop using Fluticasone Propionate Nasal Spray and see a doctor right away. |
|
|
Eye conditions such as cataracts or glaucoma |
|
Have a yearly eye exam to check for these conditions. Read the Drug Facts on the back of the Fluticasone Propionate Nasal Spray package for more details. |
|
Growth effects |
|
Talk to your doctor if you are concerned or if a child age 4-11 needs to use Fluticasone Propionate Nasal Spray for longer than two months a year. |
|
Other side effects |
|
Talk to your doctor or pharmacist if you are concerned. |
STILL HAVE QUESTIONS? Call 1-800-719-9260
FLUTICASONE PROPIONATE NASAL SPRAY
QUICK START GUIDE
HOW TO START GETTING ALLERGY RELIEF RIGHT NOW
USE THE RIGHT DOSE
Fluticasone Propionate Nasal Spray works best when you use it daily. Here’s how to get started.
Children age 4-11
An adult should supervise use
Use one spray in each nostril once daily.
Don’t use more than one spray in each nostril per day.
If you miss a dose, just use your regular dose the next day. Don’t add an extra dose.
Check with a doctor if a child needs to use Fluticasone Propionate Nasal Spray for longer than two months a year.
Users age 12 or older
In your first week
Use two sprays in each nostril every day.
Don’t use more than two sprays in each nostril per day.
After your first week
If your symptoms are under control, you may reduce to one spray in each nostril every day. If your symptoms get worse, go back to two sprays in each nostril.
If you miss a dose, just use your regular dose the next day. Don’t add an extra dose.
WARNING:
Do not spray in your eyes. Only for use in your nose.
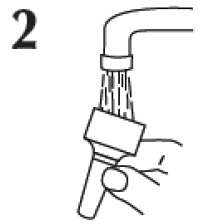
KEEP IT CLEAN
A clean spray nozzle helps ensure a full dose. Clean it weekly, or if it’s clogged. Don’t try to unblock nozzle with pin or sharp object — that can damage it.
Remove spray nozzle by grasping at base and pulling up.
Rinse under running tap, and dry at room temperature.
Aim away from your face and gently replace spray nozzle until you hear a soft click.
If spray nozzle is clogged, soak in warm water. Then repeat steps 2 and 3.
GET THE RELIEF YOU NEED.
Fluticasone Propionate Nasal Spray can relieve your allergy symptoms. For best results, it’s important to get a full dose.
Here’s how, in five easy steps.
Gently shake spray bottle.
Remove clear cap.
Do this when:
- •
- Starting new bottle
- •
- Haven’t used it in a week
- •
- Just cleaned nozzle
Otherwise go to Step 3.
Aim away from face. Grasp spray bottle as shown. Pump until fine mist appears.
Pumped six times and still no mist? Spray nozzle may be clogged. See KEEP IT CLEAN.
Blow nose gently to clear nostrils.
Close one nostril and put tip of spray nozzle in other nostril.
Put just the tip into your nose.
Aim slightly away from center of nose.
While sniffing gently, press down on spray nozzle once or twice (according to dosing instructions). You’ll feel a light mist in your nose. Breathe out through your mouth.
Repeat in other nostril.
Wipe spray nozzle with clean tissue and replace cap.
Distributed By
Perrigo®
Allegan, MI 49010
1G700 00 J5:
1G700 00 J6:
Package/Label Principal Display Panel
Allergy Relief
Children’s 24-Hour Allergy Nasal Spray
Fluticasone Propionate Nasal Spray 50 mcg Per Spray
Allergy Symptom Reliever (Glucocorticoid)*
24 HOUR RELIEF
24 Hour Relief of:
Itchy, Watery Eyes - Nasal Congestion
Runny Nose - Itchy Nose - Sneezing
Ages 4 Years & Older
Full Prescription Strength
Non-Drowsy
Compare to active ingredient of Children’s Flonase® Allergy Relief
72 Metered Sprays
0.38 FL OZ (11.1mL)
* Fluticasone propionate is a steroid medicine known as a glucocorticoid.
INGREDIENTS AND APPEARANCE
| GOOD SENSE 24 HOUR ALLERGY NASAL
fluticasone propionate spray, metered |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - L. Perrigo Company (006013346) |