Search by Drug Name or NDC
NDC 00113-1723-03 Good Sense Omeprazole 20 mg/1 Details
Good Sense Omeprazole 20 mg/1
Good Sense Omeprazole is a ORAL TABLET, DELAYED RELEASE in the HUMAN OTC DRUG category. It is labeled and distributed by L. Perrigo Company. The primary component is OMEPRAZOLE.
MedlinePlus Drug Summary
Prescription omeprazole is used alone or with other medications to treat the symptoms of gastroesophageal reflux disease (GERD), a condition in which backward flow of acid from the stomach causes heartburn and possible injury of the esophagus (the tube between the throat and stomach) in adults and children 1 year of age and older. Prescription omeprazole is used to treat damage from GERD in adults and children 1 month of age and older. Prescription omeprazole is used to allow the esophagus to heal and prevent further damage to the esophagus in adults and children 1 year of age and older with GERD. Prescription omeprazole is also used to treat conditions in which the stomach produces too much acid such as Zollinger-Ellison syndrome in adults. Prescription omeprazole is also used to treat ulcers (sores in the lining of the stomach or intestine) and it is also used with other medications to treat and prevent the return of ulcers caused by a certain type of bacteria (H. pylori) in adults. Nonprescription (over-the-counter) omeprazole is used to treat frequent heartburn (heartburn that occurs at least 2 or more days a week) in adults. Omeprazole is in a class of medications called proton-pump inhibitors. It works by decreasing the amount of acid made in the stomach.
Related Packages: 00113-1723-03Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Omeprazole
Product Information
| NDC | 00113-1723 |
|---|---|
| Product ID | 0113-1723_2726e5fd-0cd1-45b8-900b-1c296e9a794e |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Good Sense Omeprazole |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Omeprazole |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, DELAYED RELEASE |
| Route | ORAL |
| Active Ingredient Strength | 20 |
| Active Ingredient Units | mg/1 |
| Substance Name | OMEPRAZOLE |
| Labeler Name | L. Perrigo Company |
| Pharmaceutical Class | Cytochrome P450 2C19 Inhibitors [MoA], Proton Pump Inhibitor [EPC], Proton Pump Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA022032 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

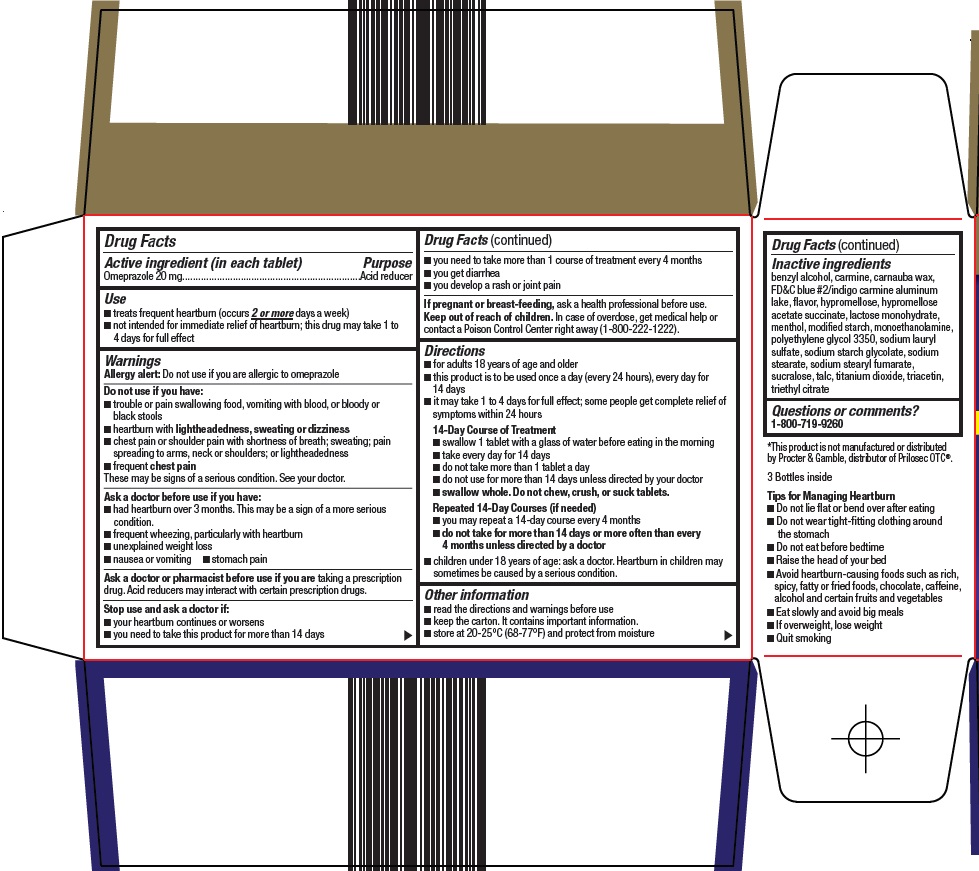
NDC 00113-1723-03 (00113172303)
| NDC Package Code | 0113-1723-03 |
|---|---|
| Billing NDC | 00113172303 |
| Package | 3 BOTTLE in 1 CARTON (0113-1723-03) / 14 TABLET, DELAYED RELEASE in 1 BOTTLE |
| Marketing Start Date | 2019-05-22 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL db333c49-2c3e-4ade-a202-7d4499205fc4 Details
Use
Do not use if you have:
- •
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have:
Ask a doctor or pharmacist before use if you are
Stop use and ask a doctor if:
Keep out of reach of children.
Directions
- •
- for adults 18 years of age and older
- •
- this product is to be used once a day (every 24 hours), every day for 14 days
- •
- it may take 1 to 4 days for full effect; some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
- •
- swallow 1 tablet with a glass of water before eating in the morning
- •
- take every day for 14 days
- •
- do not take more than 1 tablet a day
- •
- do not use for more than 14 days unless directed by your doctor
- •
- swallow whole. Do not chew, crush, or suck tablets.
Repeated 14-Day Courses (if needed)
- •
- you may repeat a 14-day course every 4 months
- •
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- •
- children under 18 years of age: ask a doctor. Heartburn in children may sometimes be caused by a serious condition.
Other information
Inactive ingredients
benzyl alcohol, carmine, carnauba wax, FD&C blue #2/indigo carmine aluminum lake, flavor, hypromellose, hypromellose acetate succinate, lactose monohydrate, menthol, modified starch, monoethanolamine, polyethylene glycol 3350, sodium lauryl sulfate, sodium starch glycolate, sodium stearate, sodium stearyl fumarate, sucralose, talc, titanium dioxide, triacetin, triethyl citrate
Package/Label Principal Display Panel
INGREDIENTS AND APPEARANCE
| GOOD SENSE OMEPRAZOLE
omeprazole tablet, delayed release |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - L. Perrigo Company (006013346) |