Search by Drug Name or NDC
NDC 00169-7705-92 Norditropin 10 mg/1.5mL Details
Norditropin 10 mg/1.5mL
Norditropin is a SUBCUTANEOUS INJECTION, SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Novo Nordisk. The primary component is SOMATROPIN.
MedlinePlus Drug Summary
Somatropin injection is used to replace growth hormone (a natural hormone produced by your body) in adults and children with growth hormone deficiency. Somatropin injection is also used to increase growth in children with certain conditions that affect normal growth and development. Somatropin injection (Serostim) is used to increase body weight and physical endurance in patients with human immunodeficiency virus (HIV) who have HIV-associated wasting syndrome. Somatropin injection (Zorbtive) is used to treat short bowel syndrome in adults who are receiving additional nutrition or fluids from intravenous (IV) therapy. Somatropin is a human growth hormone (hGH) analog. It works by replacing growth hormones that are normally produced in the body, which may result in increased growth, body weight, and improved absorption of nutrients and fluids from the intestines.
Related Packages: 00169-7705-92Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Somatropin
Product Information
| NDC | 00169-7705 |
|---|---|
| Product ID | 0169-7705_f29c93fa-6486-465b-88a9-6c2d984d17f9 |
| Associated GPIs | 3010002000D230 |
| GCN Sequence Number | 058668 |
| GCN Sequence Number Description | somatropin PEN INJCTR 10MG/1.5ML SUBCUT |
| HIC3 | P1A |
| HIC3 Description | GROWTH HORMONES |
| GCN | 24146 |
| HICL Sequence Number | 002824 |
| HICL Sequence Number Description | SOMATROPIN |
| Brand/Generic | Brand |
| Proprietary Name | Norditropin |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | somatropin |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, SOLUTION |
| Route | SUBCUTANEOUS |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/1.5mL |
| Substance Name | SOMATROPIN |
| Labeler Name | Novo Nordisk |
| Pharmaceutical Class | Human Growth Hormone [CS], Recombinant Human Growth Hormone [EPC] |
| DEA Schedule | n/a |
| Marketing Category | BLA |
| Application Number | BLA021148 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images




NDC 00169-7705-92 (00169770592)
| NDC Package Code | 0169-7705-92 |
|---|---|
| Billing NDC | 00169770592 |
| Package | 1 SYRINGE, PLASTIC in 1 CARTON (0169-7705-92) / 1.5 mL in 1 SYRINGE, PLASTIC |
| Marketing Start Date | 2010-03-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 1058e17c-9261-459c-a3e6-fae38d196c14 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
NORDITROPIN ® (somatropin) injection, for subcutaneous use
Initial U.S. Approval: 1987
INDICATIONS AND USAGE
NORDITROPINis a recombinant human growth hormone indicated for:
- •
- Pediatric: Treatment of pediatric patients with growth failure due to inadequate secretion of endogenous growth hormone (GH), short stature associated withNoonan syndrome, short stature associated with Turner syndrome, short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years, Idiopathic Short Stature (ISS), and growth failure due to Prader-Willi Syndrome (1.1)
- •
- Adult: Replacement of endogenous GH in adults with growth hormone deficiency (1.2)
DOSAGE AND ADMINISTRATION
- •
- Administer by subcutaneous injection to the back of upper arm, abdomen, buttock, or thigh with regular rotation of injection sites (2.1)
- •
- Pediatric Dosage - divide the calculated weekly dosage into equal doses given either 6, or 7 days per week
- •
-
Adult Dosage:Either of the following two dosing regimens may be used:
- o
- Non-weight based dosing: Initiate with a dose of approximately 0.2 mg/day (range, 0.15 mg/day-0.3 mg/day) and increase the dose every 1-2 months by increments of approximately 0.1 mg/day-0.2 mg/day, according to individual patient requirements (2.3)
- o
- Weight-based dosing (Not recommended for obese patients): Initiate at 0.004 mg/kg daily and increase the dose according to individual patient requirements to a maximum of 0.016 mg/kg daily (2.3)
DOSAGE FORMS AND STRENGTHS
NORDITROPIN injectionis available as (3):
- •
- 5 mg/1.5 mL (orange): FlexPro single-patient-use pen
- •
- 10 mg/1.5 mL (blue): FlexPro single-patient-use pen
- •
- 15 mg/1.5 mL (green): FlexPro single-patient-use pen
- •
- 30 mg/3 mL (purple): FlexPro single-patient-use pen
CONTRAINDICATIONS
- •
- Acute Critical Illness (4)
- •
- Pediatric patients with Prader-Willi syndrome who are severely obese, have history of severe upper airway obstruction, or have severe respiratory impairment due to risk of sudden death (4)
- •
- Active Malignancy (4)
- •
- Hypersensitivity to somatropin or excipients (4)
- •
- Active Proliferative or Severe Non-Proliferative Diabetic Retinopathy (4)
- •
- Pediatric patients with closed epiphyses (4)
WARNINGS AND PRECAUTIONS
- •
- Increased Risk of Neoplasms: Second neoplasms have occurred in childhood cancer survivors. Monitor patients with preexisting tumors for progression or recurrence. (5.3)
- •
- Glucose Intolerance and Diabetes Mellitus: NORDITROPIN may decrease insulin sensitivity, particularly at higher doses. Monitor glucose levels periodically in all patients receiving NORDITROPIN, especially in patients with existing diabetes mellitus or at risk for development. (5.4)
- •
- Intracranial Hypertension (IH): Has been reported usually within 8 weeks of initiation. Perform fundoscopic examinations prior to initiation and periodically thereafter. If papilledema occurs, stop treatment. (5.5)
- •
- Severe Hypersensitivity: Serious hypersensitivity reactions may occur. In the event of an allergic reaction, seek prompt medical attention. (5.6)
- •
- Fluid Retention: May occur in adults and may be dose dependent. (5.7)
- •
- Hypoadrenalism: Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increases in those with known hypoadrenalism. (5.8)
- •
- Hypothyroidism: Monitor thyroid function periodically as hypothyroidism may occur or worsen after initiation of somatropin. (5.9)
- •
- Slipped Capital Femoral Epiphysis in Pediatric Patients: May occur; evaluate patients with onset of a limp or hip/knee pain. (5.10)
- •
- Progression of Preexisting Scoliosis in Pediatric Patients: Monitor patients with scoliosis for progression. (5.11)
- •
- Pancreatitis: Has been reported; consider pancreatitis in patients with abdominal pain, especially pediatric patients. (5.12)
ADVERSE REACTIONS
Common adverse reactions in adult and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk at 1-888-NOVO-444 (1-888-668-6444) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of NORDITROPIN (7)
- •
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth. (7)
- •
- Cytochrome P450-Metabolized Drugs: NORDITROPIN may alter the clearance. Monitor carefully if used with NORDITROPIN (7)
- •
- Oral Estrogen: Larger doses of NORDITROPIN may be required (7)
- •
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required (5.4, 7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2020
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pediatric Patients
1.2 Adult Patients
2 DOSAGE AND ADMINISTRATION
2.1 Administration and Use Instructions
2.2 Pediatric Dosage
2.3 Adult Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Patients with Acute Critical Illness
5.2 Sudden Death in Pediatric Patients with Prader-Willi Syndrome
5.3 Increased Risk of Neoplasms
5.4 Glucose Intolerance and Diabetes Mellitus
5.5 Intracranial Hypertension
5.6 Severe Hypersensitivity
5.7 Fluid Retention
5.8 Hypoadrenalism
5.9 Hypothyroidism
5.10 Slipped Capital Femoral Epiphysis in Pediatric Patients
5.11 Progression of Preexisting Scoliosis in Pediatric Patients
5.12 Pancreatitis
5.13 Lipoatrophy
5.14 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Growth Failure due to Inadequate Secretion of Endogenous Growth Hormone
14.2 Short Stature Associated with Noonan Syndrome
14.3 Short Stature Associated with Turner Syndrome
14.4 Short Stature in Children Born Small for Gestational Age (SGA) with No Catch-up Growth by Age 2-4 Years
14.5 Idiopathic Short Stature (ISS)
14.6 Growth Failure Due to Prader-Willi Syndrome (PWS)
14.7 Adults with Growth Hormone Deficiency (GHD)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
1.1 Pediatric Patients
NORDITROPIN is indicated for the treatment of pediatric patients with:
- •
- growth failure due to inadequate secretion of endogenous growth hormone (GH),
- •
- short stature associated with Noonan syndrome,
- •
- short stature associated with Turner syndrome,
- •
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 years to 4 years of age,
- •
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range,
- •
- growth failure due to Prader-Willi syndrome (PWS).
2 DOSAGE AND ADMINISTRATION
2.1 Administration and Use Instructions
- •
- Therapy with NORDITROPIN should be supervised by a physician who is experienced in the diagnosis and management of patients with the conditions for which NORDITROPIN is indicated [see Indications and Usage (1)].
- •
- Fundoscopic examination should be performed routinely before initiating treatment with NORDITROPIN to exclude preexisting papilledema, and periodically thereafter [see Warnings and Precautions (5.5)].
- •
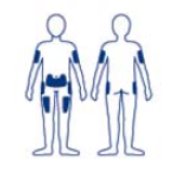
- Administer NORDITROPIN by subcutaneous injection to the back of the upper arm, abdomen, buttocks, or thigh with regular rotation of injection sites to avoid lipoatrophy.
- •
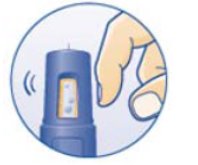
- Inspect visually for particulate matter and discoloration. NORDITROPIN should be clear and colorless. If the solution is cloudy or contains particulate matter do not use.
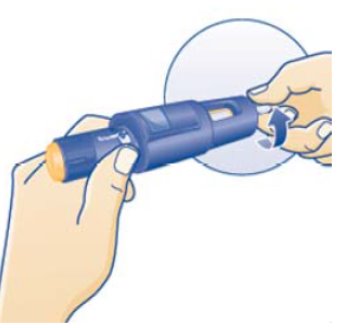
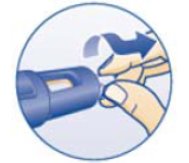
- •
- Instructions for delivering the dosage are provided in the PATIENT INFORMATION and INSTRUCTIONS FOR USE leaflets enclosed with the NORDITROPIN FlexPro prefilled pen.
2.2 Pediatric Dosage
- •
- Individualize dosage for each patient based on the growth response.
- •
- Divide the calculated weekly NORDITROPIN dosage into equal doses given either 6, or 7 days per week.
- •
- The recommended weekly dose in milligrams (mg) per kilogram (kg) of body weight for pediatric patients is:
- o
- Pediatric GH Deficiency: 0.17 mg/kg/week to 0.24 mg/kg/week (0.024 to 0.034 mg/kg/day)
- o
- Noonan Syndrome: Up to 0.46 mg/kg/week (up to 0.066 mg/kg/day)
- o
- Turner Syndrome: Up to 0.47 mg/kg/week (up to 0.067 mg/kg/day)
- o
- Small for Gestational Age (SGA): Up to 0.47 mg/kg/week (up to 0.067 mg/kg/day)
- ▪
- In very short pediatric patients, HSDS less than -3, and older pubertal pediatric patients consider initiating treatment with a larger dose of NORDITROPIN (up to 0.067 mg/kg/day). Consider a gradual reduction in dosage if substantial catch-up growth is observed during the first few years of therapy. In pediatric patients less than 4 years of age with less severe short stature, baseline HSDS values between -2 and -3, consider initiating treatment at 0.033 mg/kg/day and titrate the dose as needed.
- o
- Idiopathic Short Stature: Up to 0.47 mg/kg/week (up to 0.067 mg/kg/day)
- o
- Prader-Willi Syndrome: 0.24 mg/kg/week (0.034 mg/kg/day)
- •
- Assess compliance and evaluate other causes of poor growth such as hypothyroidism, under-nutrition, advanced bone age and antibodies to recombinant human growth hormone if patients experience failure to increase height velocity, particularly during the first year of treatment.
- •
- Discontinue NORDITROPIN for stimulation of linear growth once epiphyseal fusion has occurred [see Contraindications (4)].
2.3 Adult Dosage
- •
- Patients who were treated with somatropin for GH deficiency in childhood and whose epiphyses are closed should be reevaluated before continuation of somatropin for GH deficient adults.
- •
- Consider using a lower starting dose and smaller dose increment increases for geriatric patients as they may be at increased risk for adverse reactions with NORDITROPIN than younger individuals [see Use in Specific Populations (8.5)].
- •
- Estrogen-replete women and patients receiving oral estrogen may require higher doses [see Drug Interactions (7)].
- •
- Administer the prescribed dose daily.
- •
- Either of two NORDITROPIN dosing regimens may be used:
- o
- Non-weight based
- ▪
- Initiate NORDITROPIN with a dose of approximately 0.2 mg/day (range, 0.15 mg/day to 0.3 mg/day) and increase the dose every 1-2 months by increments of approximately 0.1 mg/day to 0.2 mg/day, according to individual patient requirements based on the clinical response and serum insulin-like growth factor 1 (IGF-1) concentrations.
- ▪
- Decrease the dose as necessary on the basis of adverse reactions and/or serum IGF-1 concentrations above the age- and gender-specific normal range.
- ▪
- Maintenance dosages will vary considerably from person to person, and between male and female patients.
- o
- Weight-based
- ▪
- Initiate NORDITROPIN at 0.004 mg/kg daily and increase the dose according to individual patient requirements to a maximum of 0.016 mg/kg daily.
- ▪
- Use the patient’s clinical response, adverse reactions, and determination of age- and gender-adjusted serum IGF-1 concentrations as guidance in dose titration.
- ▪
- Not recommended for obese patients as they are more likely to experience adverse reactions with this regimen
3 DOSAGE FORMS AND STRENGTHS
NORDITROPINinjection is a clear and colorless solution available as follows:
- •
- 5 mg in 1.5 mL (orange): NORDITROPINFlexPro single-patient-use pen
- •
- 10 mg in 1.5 mL (blue): NORDITROPIN FlexPro single-patient-use pen
- •
- 15 mg in 1.5 mL (green): NORDITROPIN FlexPro single-patient-use pen
- •
- 30 mg in 3 mL (purple): NORDITROPIN FlexPro single-patient-use pen
4 CONTRAINDICATIONS
NORDITROPIN is contraindicated in patients with:
- •
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin [see Warnings and Precautions (5.1)].
- •
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death [see Warnings and Precautions (5.2)].
- •
- Active Malignancy [see Warnings and Precautions (5.3)].
- •
- Hypersensitivity to NORDITROPIN or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropin products [see Warnings and Precautions (5.6)].
- •
- Active proliferative or severe non-proliferative diabetic retinopathy.
- •
- Pediatric patients with closed epiphyses.
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Patients with Acute Critical Illness
Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure has been reported after treatment with pharmacologic amounts of somatropin [see Contraindications (4)]. Two placebo-controlled clinical trials in non-growth hormone deficient adult patients (n=522) with these conditions in intensive care units revealed a significant increase in mortality (42% vs. 19%) among somatropin-treated patients (doses 5.3-8 mg/day) compared to those receiving placebo. The safety of continuing NORDITROPINtreatment in patients receiving replacement doses for approved indications who concurrently develop these illnesses has not been established. NORDITROPIN is not indicated for the treatment of non-GH deficient adults.
5.2 Sudden Death in Pediatric Patients with Prader-Willi Syndrome
There have been reports of sudden death after initiating therapy with somatropin in pediatric patients with Prader-Willi syndrome who had one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than females. Patients with Prader-Willi syndrome should be evaluated for signs of upper airway obstruction and sleep apnea before initiation of treatment with somatropin. If, during treatment with NORDITROPIN, patients show signs of upper airway obstruction (including onset of or increased snoring) and/or new onset sleep apnea, treatment should be interrupted. All patients with Prader-Willi syndrome treated with NORDITROPINshould also have effective weight control and be monitored for signs of respiratory infection, which should be diagnosed as early as possible and treated aggressively [see Contraindications (4)].
5.3 Increased Risk of Neoplasms
Active Malignancy
There is an increased risk of malignancy progression with somatropin treatment in patients with active malignancy [See Contraindications (4)]. Any preexisting malignancy should be inactive and its treatment complete prior to instituting therapy with NORDITROPIN. Discontinue NORDITROPIN if there is evidence of recurrent activity.
Risk of Second Neoplasm in Pediatric Patients
There is an increased risk of a second neoplasm in pediatric cancer survivors who were treated with radiation to the brain/head and who developed subsequent GH deficiency and were treated with somatropin. Intracranial tumors, in particular meningiomas, were the most common of these second neoplasms. In adults, it is unknown whether there is any relationship between somatropin replacement therapy and CNS tumor recurrence. Monitor all patients receiving NORDITROPIN who have a history of GH deficiency secondary to an intracranial neoplasm for progression or recurrence of the tumor.
New Malignancy During Treatment
Because pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies, thoroughly consider the risks and benefits of starting NORDITROPIN in these patients. If NORDITROPIN is initiated, carefully monitor patients for development of neoplasms.
Monitor all patients receiving NORDITROPIN carefully for increased growth, or potential malignant changes, of preexisting nevi. Advise patients/caregivers to report marked changes in behavior, onset of headaches, vision disturbances and/or changes in skin pigmentation or changes in the appearance of pre-existing nevi.
5.4 Glucose Intolerance and Diabetes Mellitus
Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New onset type 2 diabetes mellitus has been reported in patients taking somatropin. Previously undiagnosed impaired glucose tolerance and overt diabetes mellitus may be unmasked. Monitor glucose levels periodically in all patients receiving NORDITROPIN, especially in those with risk factors for diabetes mellitus, such as obesity, Turner syndrome, or a family history of diabetes mellitus. Patients with preexisting type 1 or type 2 diabetes mellitus or impaired glucose tolerance should be monitored closely. The doses of antidiabetic agents may require adjustment when NORDITROPIN is initiated.
5.5 Intracranial Hypertension
Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea, and/or vomiting has been reported in a small number of patients treated with somatropin products. Symptoms usually occurred within the first eight (8) weeks after the initiation of somatropin therapy. In all reported cases, IH-associated signs and symptoms rapidly resolved after cessation of therapy or a reduction of the somatropin dose. Funduscopic examination should be performed routinely before initiating treatment with NORDITROPIN to exclude preexisting papilledema, and periodically thereafter. If papilledema is observed by funduscopy during somatropin treatment, treatment should be stopped. If somatropin-induced IH is diagnosed, treatment with NORDITROPIN can be restarted at a lower dose after IH-associated signs and symptoms have resolved. Patients with Turner syndrome may be at increased risk for the development of IH.
5.6 Severe Hypersensitivity
Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropin products. Patients and caregivers should be informed that such reactions are possible and that prompt medical attention should be sought if an allergic reaction occurs [see Contraindications (4)].
5.7 Fluid Retention
Fluid retention during somatropin replacement therapy in adults may frequently occur. Clinical manifestations of fluid retention (e.g. edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) are usually transient and dose dependent.
5.8 Hypoadrenalism
Patients receiving somatropin therapy who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of NORDITROPINtreatment. Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increases in those with known hypoadrenalism [see Drug Interactions (7)].
5.9 Hypothyroidism
Undiagnosed/untreated hypothyroidism may prevent an optimal response to NORDITROPIN, in particular, the growth response in pediatric patients. Patients with Turner syndrome have an inherently increased risk of developing autoimmune thyroid disease and primary hypothyroidism. In patients with GH deficiency, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Therefore, patients should have periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or appropriately adjusted when indicated.
5.10 Slipped Capital Femoral Epiphysis in Pediatric Patients
Slipped capital femoral epiphysis may occur more frequently in patients with endocrine disorders (including GH deficiency and Turner syndrome) or in patients undergoing rapid growth. Evaluate pediatric patients with the onset of a limp or complaints of hip or knee pain.
5.11 Progression of Preexisting Scoliosis in Pediatric Patients
Somatropin increases the growth rate, and progression of existing scoliosis can occur in patients who experience rapid growth. Somatropin has not been shown to increase the occurrence of scoliosis. Monitor patients with a history of scoliosis for progression of scoliosis.
5.12 Pancreatitis
Cases of pancreatitis have been reported in pediatric patients and adults receiving somatropin products. There may be a greater risk in pediatric patients compared with adults. Published literature indicates that females who have Turner syndrome may be at greater risk than other pediatric patients receiving somatropin products. Pancreatitis should be considered in patients who develop persistent severe abdominal pain.
5.13 Lipoatrophy
When somatropin products are administered subcutaneously at the same site over a long period of time, tissue atrophy may result. Rotate injection sites when administering NORDITROPIN to reduce this risk [see Administration and Use Instructions (2.1)].
6 ADVERSE REACTIONS
The following important adverse reactions are also described elsewhere in the labeling:
- •
- Increased mortality in patients with acute critical illness [see Warnings and Precautions (5.1)]
- •
- Sudden death in children with Prader-Willi syndrome [see Warnings and Precautions (5.2)]
- •
- Neoplasms [see Warnings and Precautions (5.3)]
- •
- Glucose intolerance and diabetes mellitus [see Warnings and Precautions (5.4)]
- •
- Intracranial hypertension [see Warnings and Precautions (5.5)]
- •
- Severe hypersensitivity [see Warnings and Precautions (5.6)]
- •
- Fluid retention [see Warnings and Precautions (5.7)]
- •
- Hypoadrenalism [see Warnings and Precautions (5.8)]
- •
- Hypothyroidism [see Warnings and Precautions (5.9)]
- •
- Slipped capital femoral epiphysis in pediatric patients [see Warnings and Precautions (5.10)]
- •
- Progression of preexisting scoliosis in pediatric patients [see Warnings and Precautions (5.11)]
- •
- Pancreatitis [see Warnings and Precautions (5.12)]
- •
- Lipoatrophy [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed during the clinical trials performed with one somatropin product cannot always be directly compared to the rates observed during the clinical trials performed with another somatropin product, and may not reflect the adverse reaction rates observed in practice.
Pediatric Patients
Growth Failure due to Inadequate Secretion of Endogenous Growth Hormone
In one randomized, open label, clinical study the most frequent adverse reactions were headache, pharyngitis, otitis media and fever. There were no clinically significant differences between the three doses assessed in the study (0.025, 0.05 and 0.1 mg/kg/day).
Short Stature Associated with Noonan Syndrome
NORDITROPINwas studied in 21 pediatric patients, 3 years to 14 years of age at doses of 0.033 mg/kg/day and 0.066 mg/kg/day. After the two-year study, patients continued NORDITROPINtreatment until final height was achieved; randomized dose groups were not maintained. Adverse reactions were later collected retrospectively from 18 pediatric patients; total follow-up was 11 years. An additional 6 pediatric patients were not randomized, but followed the protocol and are included in this assessment of adverse reactions.
The most frequent adverse reactions were upper respiratory infection, gastroenteritis, ear infection, and influenza. Cardiac disorders was the system organ class with the second most adverse reactions reported. Scoliosis was reported in 1 and 4 pediatric patients receiving doses of 0.033 mg/kg/day and 0.066 mg/kg/day respectively. The following additional adverse reactions also occurred once: insulin resistance and panic reaction for the 0.033 mg/kg/day dose group; injection site pruritus, bone development abnormal, depression, and self-injurious ideation in the 0.066 mg/kg/day dose group. Headache occurred in 2 cases in the 0.066 mg/kg/day dose group.
Short Stature Associated with Turner Syndrome
In two clinical studies in pediatric patients that were treated until final height with various doses of NORDITROPIN, the most frequently reported adverse reactions were influenza-like illness, otitis media, upper respiratory tract infection, otitis externa, gastroenteritis, eczema and, impaired fasting glucose. Adverse reactions in study 1 were most frequent in the highest dose groups. Three patients in study 1 had excessive growth of hands and/or feet in the high dose groups. Two patients in study 1 had a serious adverse reaction of exacerbation of preexisting scoliosis in the 0.045 mg/kg/day group.
Small for Gestational Age (SGA) with No Catch-up Growth by Age 2-4 Years
In a study, 53 pediatric patients were treated with 2 doses of NORDITROPIN(0.033 or 0.067 mg/kg/day) to final height for up to 13 years (mean duration of treatment 7.9 and 9.5 years for girls and boys, respectively). The most frequently reported adverse reactions were influenza-like illness, upper respiratory tract infection, bronchitis, gastroenteritis, abdominal pain, otitis media, pharyngitis, arthralgia, headache, gynecomastia, and increased sweating. One pediatric patient treated with 0.067 mg/kg/day for 4 years was reported with disproportionate growth of the lower jaw, and another patient treated with 0.067 mg/kg/day developed a melanocytic nevus. 4 pediatric patients treated with 0.067 mg/kg/day and 2 pediatric patients treated with 0.033 mg/kg/day of NORDITROPIN had increased fasting blood glucose levels after 1 year of treatment. In addition, small increases in mean fasting blood glucose and insulin levels after 1 and 2 years of NORDITROPIN treatment appeared to be dose-dependent.
In a second study, 98 Japanese pediatric patients were treated with 2 doses of NORDITROPIN(0.033 or 0.067 mg/kg/day) for 2 years or were untreated for 1 year. Adverse reactions were otitis media, arthralgia and impaired glucose tolerance. Arthralgia and transiently impaired glucose tolerance were reported in the 0.067 mg/kg/day treatment group.
Idiopathic Short Stature
In two open-label clinical studies with another somatropin product in pediatric patients, the most common adverse reactions were upper respiratory tract infections, influenza, tonsillitis, nasopharyngitis, gastroenteritis, headaches, increased appetite, pyrexia, fracture, altered mood, and arthralgia.
Growth Failure Due to Prader-Willi Syndrome
In two clinical studies in pediatric patients with PWS carried out with another somatropin product, the following adverse reactions were reported: edema, aggressiveness, arthralgia, benign intracranial hypertension, hair loss, headache, and myalgia.
Adult Patients
Adults with Growth Hormone Deficiency
Adverse reactions with an incidence of ≥5% occurring in patients with AO GHD during the 6 month placebo-controlled portion of a clinical trial for NORDITROPINare presented in Table 1.
Table 1 – Adverse Reactions with ≥5% Overall Incidence in Adult Onset Growth Hormone
Deficient Patients Treated with NORDITROPIN During a Six Month Placebo-Controlled Clinical Trial
|
Placebo (N=52) |
NORDITROPIN (N=53) |
|
|
Adverse Reactions |
% |
% |
|
Peripheral Edema |
8 |
42 |
|
Edema |
0 |
25 |
|
Arthralgia |
15 |
19 |
|
Leg Edema |
4 |
15 |
|
Myalgia |
8 |
15 |
|
Infection (non-viral) |
8 |
13 |
|
Paraesthesia |
6 |
11 |
|
Skeletal Pain |
2 |
11 |
|
Headache |
6 |
9 |
|
Bronchitis |
0 |
9 |
|
Flu-like symptoms |
4 |
8 |
|
Hypertension |
2 |
8 |
|
Gastroenteritis |
8 |
8 |
|
Other Non-Classifiable Disorders (excludes accidental injury) |
6 |
8 |
|
Increased sweating |
2 |
8 |
|
Glucose tolerance abnormal |
2 |
6 |
|
Laryngitis |
6 |
6 |
|
Type 2 diabetes mellitus |
0 |
5 |
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to NORDITROPINwith the incidence of antibodies to other products may be misleading. In the case of growth hormone, antibodies with binding capacities lower than 2 mg/mL have not been associated with growth attenuation. In a very small number of patients treated with somatropin, when binding capacity was greater than 2 mg/mL, interference with the growth response was observed.
In clinical trials, GH deficient pediatric patients receiving NORDITROPINfor up to 12 months were tested for induction of antibodies, and 0/358 patients developed antibodies with binding capacities above 2 mg/L. Amongst these patients, 165 had previously been treated with other somatropin formulations, and 193 were previously untreated naive patients. Eighteen of 76 children (~24%) treated with NORDITROPINfor short stature born SGA developed anti-rhGH antibodies.
6.3 Post-Marketing Experience
Because these adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders — Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema
Skin — Increase in size or number of cutaneous nevi
Endocrine disorders — Hypothyroidism
Metabolism and nutrition disorders — Hyperglycemia
Musculoskeletal and connective tissue disorders —Slipped capital femoral epiphysis— Legg-Calvé-Perthes disease
Investigations — Increase in blood alkaline phosphatase level — Decrease in serum thyroxin (T4) levels
Gastrointestinal — Pancreatitis
Neoplasm — Leukemia has been reported in a small number of GH deficient children treated with somatropin, somatrem (methionylated rhGH) and GH of pituitary origin
7 DRUG INTERACTIONS
Table 2 includes a list of drugs with clinically important drug interactions when administered concomitantly with NORDITROPINand instructions for preventing or managing them.
Table 2: Clinically Important Drug Interactions with NORDITROPIN
|
Glucocorticoids |
|
|
Clinical Impact: |
Microsomal enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1) is required for conversion of cortisone to its active metabolite, cortisol, in hepatic and adipose tissue. NORDITROPINinhibits 11βHSD-1. Consequently, individuals with untreated GH deficiency have relative increases in 11βHSD-1 and serum cortisol. Initiation of NORDITROPINmay result in inhibition of 11βHSD-1 and reduced serum cortisol concentrations. |
|
Intervention: |
Patients treated with glucocorticoid replacement for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of NORDITROPIN [see Warnings and Precautions (5.8)]. |
|
Examples: |
Cortisone acetate and prednisone may be effected more than others since conversion of these drugs to their biologically active metabolites is dependent on the activity of 11βHSD-1. |
|
Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment |
|
|
Clinical Impact: |
Pharmacologic glucocorticoid therapy and supraphysiologic glucocorticoid treatment may attenuate the growth promoting effects of NORDITROPINin pediatric patients. |
|
Intervention: |
Carefully adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatments to avoid both hypoadrenalism and an inhibitory effect on growth. |
|
Cytochrome P450-Metabolized Drugs |
|
|
Clinical Impact: |
Limited published data indicate that somatropin treatment increases cytochrome P450 (CP450)-mediated antipyrine clearance. NORDITROPINmay alter the clearance of compounds known to be metabolized by CP450 liver enzymes. |
|
Intervention: |
Careful monitoring is advisable when NORDITROPINis administered in combination with drugs metabolized by CP450 liver enzymes. |
|
Oral Estrogen |
|
|
Clinical Impact: |
Oral estrogens may reduce the serum IGF-1 response to NORDITROPIN. |
|
Intervention: |
Patients receiving oral estrogen replacement may require greater NORDITROPINdosages [see Dosage and Administration (2.3)]. |
|
Insulin and/or Other Hypoglycemic Agents |
|
|
Clinical Impact: |
Treatment with NORDITROPINmay decrease insulin sensitivity, particularly at higher doses. |
|
Intervention: |
Patients with diabetes mellitus may require adjustment of their doses of insulin and/or other hypoglycemic agents [see Warnings and Precautions (5.4)]. |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available data with somatropin use in pregnant women are insufficient to determine a drug-associated risk of adverse developmental outcomes. In animal reproduction studies, there was no evidence of fetal or neonatal harm when pregnant rats were administered subcutaneous NORDITROPINduring organogenesis or during lactation at doses approximately 10-times higher than the maximal clinical dose of 0.016 mg/kg, based on body surface area (see Data).
The estimated background risk of birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In an embryo-fetal development study, NORDITROPINwas administered via subcutaneous injection to pregnant rats from gestation Day 6 to 17, corresponding with the period of organogenesis. NORDITROPINdid not adversely affect fetal viability or developmental outcomes at maternal doses that were approximately 10-times the clinical dose of 0.016 mg/kg, based on body surface area.
In a pre- and post-natal development study in pregnant rats, NORDITROPINwas administered from gestation Day 17 through lactation Day 21 (weaning). No adverse developmental effects were observed in the offspring at doses up to 1.1 mg/kg (approximately 10 times the clinical dose of 0.016 mg/kg, based on body surface area).
8.2 Lactation
Risk Summary
There is no information regarding the presence of somatropin in human milk. Limited published data indicate that exogenous somatropin does not increase normal breastmilk concentrations of growth hormone. No adverse effects on the breastfed infant have been reported with somatropin. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NORDITROPINand any potential adverse effects on the breastfed infant from NORDITROPINor from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of NORDITROPIN in pediatric patients have been established in growth failure due to inadequate secretion of endogenous growth hormone, short stature associated with Noonan syndrome, short stature associated with Turner syndrome, short stature in children born small for gestational age (SGA) with no catch-up growth by age 2 years to 4 years of age, idiopathic short stature (ISS), and growth failure due to Prader-Willi syndrome (PWS).
Growth Failure due to Inadequate Secretion of Endogenous Growth Hormone
Safety and effectiveness of NORDITROPIN have been established in pediatric patients with growth failure due to growth hormone deficiency in a multi-center, prospective, randomized, open-label, dose-response study in 111 pediatric patients conducted for a two-year period [see Clinical Studies (14.1)].
Short Stature Associated with Noonan Syndrome
Safety and effectiveness of NORDITROPIN have been established in pediatric patients with Noonan syndrome in a prospective, open-label, randomized, parallel group study in 21 pediatric patients conducted for 2 years [see Clinical Studies (14.2)].
Short Stature Associated with Turner Syndrome
Safety and effectiveness of NORDITROPIN have been established in pediatric patients with short stature associated with Turner syndrome in two randomized, parallel group, open-label, multicenter studies in 87 pediatric patients [see Clinical Studies (14.3)].
Short Stature in Children Born Small for Gestational Age (SGA) with No Catch-up Growth by Age 2 Years to 4 Years of Age
Safety and effectiveness of NORDITROPIN have been established in pediatric patients with short stature born SGA with no catch-up growth in a multi-center, randomized, double-blind, two-arm study to final height in 53 pediatric patients and in a randomized study of 84 prepubertal, non-GHD, Japanese pediatric patients [see Clinical Studies (14.4)].
Idiopathic Short Stature (ISS)
Safety and effectiveness of NORDITROPIN have been established in pediatric patients with ISS based on data from a randomized, open-label clinical study with another somatropin product in 105 pediatric patients [see Clinical Studies (14.5)].
Growth Failure Due to Prader-Willi Syndrome (PWS)
Safety and effectiveness of NORDITROPIN have been established in pediatric patients with growth failure due to Prader-Willi Syndrome based on data from two randomized, open label, controlled clinical trials with another somatropin product in pediatric patients. There have been reports of sudden death after initiating therapy with somatropin in pediatric patients with Prader-Willi syndrome who had one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than females. Patients with Prader-Willi syndrome should be evaluated for signs of upper airway obstruction and sleep apnea before initiation of treatment with somatropin. [see Contraindications (4), Warnings and Precautions (5.2), Clinical Studies (14.6)].
8.5 Geriatric Use
The safety and effectiveness of NORDITROPINin patients aged 65 and over has not been evaluated in clinical studies. Elderly patients may be more sensitive to the action of somatropin, and therefore may be more prone to develop adverse reactions. A lower starting dose and smaller dose increments should be considered for older patients [see Dosage and Administration (2.3)].
9 DRUG ABUSE AND DEPENDENCE
10 OVERDOSAGE
Short-term overdosage could lead initially to hypoglycemia and subsequently to hyperglycemia. Overdose with somatropin is likely to cause fluid retention. Long-term overdosage could result in signs and symptoms of gigantism and/or acromegaly consistent with the known effects of excess growth hormone.
11 DESCRIPTION
NORDITROPIN(somatropin) for injection is a recombinant human growth hormone. It is a polypeptide of recombinant DNA origin and is synthesized by a special strain of E. coli bacteria that has been modified by the addition of a plasmid which carries the gene for human growth hormone. NORDITROPINcontains the identical sequence of 191 amino acids constituting the naturally occurring pituitary human growth hormone with a molecular weight of about 22,000 Daltons.
NORDITROPINis supplied as a sterile solution for subcutaneous use in ready-to-administer prefilled pens with a volume of 1.5 mL or 3 mL.
Each NORDITROPINcontains the following (see Table 3):
Table 3
|
Component |
5 mg/1.5 mL |
10 mg/1.5 mL |
15 mg/1.5 mL |
30 mg/3 mL |
|
Somatropin |
5 mg |
10 mg |
15 mg |
30 mg |
|
Histidine |
1 mg |
1 mg |
1.7 mg |
3.3 mg |
|
Poloxamer 188 |
4.5 mg |
4.5 mg |
4.5 mg |
9 mg |
|
Phenol |
4.5 mg |
4.5 mg |
4.5 mg |
9 mg |
|
Mannitol |
60 mg |
60 mg |
58 mg |
117 mg |
|
HCl/NaOH |
as needed |
as needed |
as needed |
as needed |
|
Water for Injection |
up to 1.5 mL |
up to 1.5 mL |
up to 1.5 mL |
up to 3 mL |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Somatropin binds to dimeric GH receptors located within the cell membranes of target tissue cells. This interaction results in intracellular signal transduction and subsequent induction of transcription and translation of GH-dependent proteins including IGF-1, IGF BP-3 and acid-labile subunit. Somatropin has direct tissue and metabolic effects or mediated indirectly by IGF-1, including stimulation of chondrocyte differentiation, and proliferation, stimulation hepatic glucose output, protein synthesis and lipolysis.
Somatropin stimulates skeletal growth in pediatric patients with GHD as a result of effects on the growth plates (epiphyses) of long bones. The stimulation of skeletal growth increases linear growth rate (height velocity) in most somatropin-treated pediatric patients. Linear growth is facilitated in part by increased cellular protein synthesis.
12.2 Pharmacodynamics
Subcutaneous administration of a single dose of 4 mg NORDITROPINin healthy subjects (n=26) with suppressed endogenous growth hormone results in an increased mean (SD) IGF-1 level from 190 (46) ng/mL predose to maximal level of 276 (49) ng/mL after approx. 24 hours. After 96 hours, the subjects displayed a mean (SD) IGF-1 concentration of 196 (41) ng/mL, comparable to the predose value.
12.3 Pharmacokinetics
Absorption - Somatropin has been studied following subcutaneous and intravenous administration in adult healthy subjects and GHD patients. A single dose administration of 4 mg NORDITROPINin healthy subjects (n=26) with suppressed endogenous growth hormone resulted in a mean (SD) Cmax of 34.9 (10.4) ng/mL after approximately 3.0 hours. After a 180-min IV infusion of NORDITROPIN(33 ng/kg/min) administered to GHD patients (n=9), a mean (SD) hGH steady state serum level of approximately 23.1 (15.0) ng/mL was reached at 150 min.
After a SC dose of 0.024 mg/kg or 3 IU/m2 given in the thigh to adult GHD patients (n=18), mean (SD) Cmax values of 13.8 (5.8) and 17.1 (10.0) ng/mL were observed for the 4 and 8 mg NORDITROPINvials, respectively, at approximately 4 to 5 hr. post dose. The absolute bioavailability for NORDITROPINafter the SC route of administration is currently not known.
Distribution— The mean (SD) apparent volume of distribution of somatropin after single dose subcutaneous administration of 4 mg NORDITROPINin healthy subjects is 43.9 (14.9) L.
Elimination
Metabolism — Extensive metabolism studies have not been conducted. The metabolic fate of somatropin involves classical protein catabolism in both the liver and kidneys.
Excretion – The mean apparent terminal T1/2 values in healthy adult subjects (n=26) was 2.0 (0.5) hours. In GHD patients receiving 180-min IV infusion of NORDITROPIN(33 ng/kg/min), a mean clearance rate of approximately 2.3 (1.8) mL/min/kg or 139 (105) mL/min for hGH was observed. Following infusion, serum hGH levels had a biexponential decay with a terminal elimination half-life (T1/2) of approximately 21.1 (5.1) min. The mean apparent terminal T1/2 values in GHD patients receiving a SC dose of 0.024 mg/kg or 3 IU/m2 was estimated to be approximately 7 to 10 hr. The longer half-life observed after subcutaneous administration is due to slow absorption from the injection site. Urinary excretion of intact somatropin has not been measured.
Geriatric patients — The pharmacokinetics of somatropin have not been studied in patients greater than 65 years of age.
Pediatric patients — The pharmacokinetics of somatropin in pediatric patients are similar to those of adults.
Male and Female Patients — No gender-specific pharmacokinetic studies have been performed with somatropin. The available literature indicates that the pharmacokinetics of somatropin are similar in men and women.
Patients with Renal or Hepatic Impairment — No studies have been performed with somatropin.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with NORDITROPIN.
Impairment of Fertility
In a rat study evaluating female fertility, animals were administered once daily subcutaneous doses of 0.1, 0.3, and 1.1 mg/kg NORDITROPIN beginning two weeks prior to mating, throughout mating and during the first 7 days of pregnancy. Delays in the time to mating were observed at doses greater than or equal to 0.3 mg/kg (approximately 3 times the maximum adult clinical dose of 0.016 mg/kg, based on body surface area), but these doses were also associated with increases in the number of corpora lutea and implantations. A decrease in the pregnancy rate was observed at the dose of 1.1 mg/kg (approximately 10 times the clinical dose of 0.016 mg/kg, based on body surface area). Male fertility parameters have not been evaluated with administration of NORDITROPIN.
14 CLINICAL STUDIES
14.1 Growth Failure due to Inadequate Secretion of Endogenous Growth Hormone
The efficacy and safety of NORDITROPIN was assessed in a multicenter, prospective randomized, open label, dose response study with three doses (0.025, 0.05 and 0.1 mg/kg/day). A total of 111 pediatric patients with GH deficiency were randomized to each dose; 37(0.025 mg/kg/day):38(0.05 mg/kg/day):36(0.1 mg/kg/day). Patients met the following entry criteria: chronological age ≥ 3 years with a skeletal age < 10 years if male and < 8 years if female; pubertal stage = stage 1; previously untreated GH deficiency; peak plasma hormone concentration < 7 ng/ml or < 10 ng/ml (depending on assay used) in two tests.
The results are displayed in Table 4. The adjusted mean increases in HSDS over the 2-year period were 0.81, 1.57 and 1.73 in the 0.025, 0.05 and 0.1 mg/kg/day dose groups, respectively. There was no significant difference in ΔHSDS between the 0.05 and 0.1 mg/kg/day treatment groups. Height velocity (HV, cm/year) and HVSDS increased considerably after initiation of treatment, with the greatest response observed during the first year of treatment.
Table 4 – Efficacy of NORDITROPIN in Pediatric GH Deficiency
|
NORDITROPIN |
|||||||||
|
0.025 mg/kg/day |
0.05 mg/kg/day |
0.1 mg/kg/day |
|||||||
|
N |
Mean |
SD |
N |
Mean |
SD |
N |
Mean |
SD |
|
|
Change in Standing Height (cm) | |||||||||
|
Baseline to Month 12 |
37 |
9.5 |
2.1 |
37 |
13.2* |
2.6 |
34 |
13.7* |
2.7 |
|
Baseline to Month 24 |
34 |
17.6 |
3.4 |
37 |
22.2* |
4.7 |
33 |
23.7* |
4.0 |
|
Change in Sitting Height (cm) | |||||||||
|
Baseline to Month 12 |
32 |
5.4 |
2.4 |
36 |
6.5* |
1.6 |
32 |
7.4** |
1.5 |
|
Baseline to Month 24 |
29 |
9.3 |
2.5 |
35 |
10.8** |
2.6 |
31 |
12.2** |
2.0 |
|
Change in Bone Age (yr) | |||||||||
|
Baseline to Month 12 |
37 |
1.3 |
0.9 |
38 |
1.7 |
0.8 |
34 |
1.6 |
0.8 |
|
Month 12 to Month 24 |
37 |
0.6 |
2.5 |
38 |
1.4 |
2.7 |
34 |
1.6* |
0.8 |
- *Significant (p <0.05) change from baseline compared to the 0.025 mg/kg/group
**Significant (p <0.05) change from baseline compared to both other groups
14.2 Short Stature Associated with Noonan Syndrome
A prospective, open label, randomized, parallel group study with 21 pediatric patients was conducted for 2 years to evaluate the efficacy and safety of NORDITROPIN. Additional 6 children were not randomized, but did follow the protocol. Inclusion criteria included bone age determination showing no significant acceleration, prepubertal status, height SDS <-2, and HV SDS <1 during the 12 months pre-treatment. Exclusion criteria were previous or ongoing treatment with growth hormone, anabolic steroids or corticosteroids, congenital heart disease or other serious disease perceived to possibly have major impact on growth, FPG >6.7 mmol/L (>120 mg/dL), or growth hormone deficiency (peak GH levels <10 ng/mL). The twenty-four, 12 female and 12 male, patients 3 – 14 years of age received either 0.033 mg/kg/day or 0.066 mg/kg/day of NORDITROPINsubcutaneously which was adjusted based on growth response after the first 2 years.
After the initial two-year study, NORDITROPIN treatment continueduntil final height. Retrospective final height was collected from 18 patients in the study and the 6 who had followed the protocol without randomization. Historical reference materials of height velocity and adult height analyses of Noonan patients served as the controls.
Patients obtained a final height (FH) gain from baseline of 1.5 and 1.6 SDS estimated according to the national and the Noonan reference, respectively. A height gain of 1.5 SDS (national) corresponds to a mean height gain of 9.9 cm in boys and 9.1 cm in girls at 18 years of age, while a height gain of 1.6 SDS (Noonan) corresponds to a mean height gain of 11.5 cm in boys and 11.0 cm in girls at 18 years of age.
A comparison of HV between the two treatment groups during the first two years of treatment for the randomized subjects was 10.1 and 7.6 cm/year with 0.066 mg/kg/day versus 8.55 and 6.7 cm/year with 0.033 mg/kg/day, for Year 1 and Year 2, respectively.
14.3 Short Stature Associated with Turner Syndrome
Two randomized, parallel group, open label, multicenter studies were conducted in the Netherlands to evaluate the efficacy and safety of NORDITROPIN. Patients were treated to final height in both studies [height velocity (HV) < 2 cm/year]. Changes in height were expressed as standard deviation scores (SDS) utilizing reference data for untreated Turner syndrome patients as well as the national Dutch population.
In Study 1, 68 euthyroid Caucasian patients stratified based on age and baseline height SDS were randomized in a 1:1:1 ratio to three different NORDITROPINtreatment regimens: 0.045 mg/kg/day (Dose A) for the entire study; 0.045 mg/kg/day for the first year and 0.067 mg/kg/day thereafter (Dose B); or 0.045 mg/kg/day for the first year, 0.067 for the second year, and 0.089 mg/kg/day thereafter (Dose C). At baseline, mean age was 6.5 years, mean height SDS (National standard) was -2.7, and mean HV during the previous year was 6.5 cm/year. Patients also received estrogen therapy after age 12 and following four years of NORDITROPINtreatment if they did not have spontaneous puberty.
Patients were treated for a mean of 8.4 years. As seen in Table 5, overall mean final height was 161 cm in the 46 children who attained final height. Seventy percent of these children reached a final height within the normal range (height SDS > -2 using the National standard). A greater percentage of children in the two escalated dose groups reached normal final height. The mean changes from baseline to final height in height SDS after treatment with Dose B and Dose C were significantly greater than the mean changes observed after treatment with Dose A (utilizing both the National and Turner standards). The mean changes from baseline to final height in height SDS (Turner standard) in Table 5 correspond to mean height gains of 9.4, 14.1 and 14.4 cm after treatment with Doses A, B and C, respectively. The mean changes from baseline to final height in height SDS (National standard) in Table 5 correspond to mean height gains of 4.5, 9.1 and 9.4 cm after treatment with Doses A, B and C, respectively. In each treatment group, peak HV was observed during treatment Year 1, and then gradually decreased each year; during Year 4, HV was less than the pre-treatment HV. However, between Year 2 and Year 6, a greater HV was observed in the two dose escalation groups compared to the 0.045 mg/kg/day group.
Table 5 – Final Height-Related Results After Treatment of Patients with Turner Syndrome with NORDITROPIN in a Randomized, Dose Escalating Study
|
Dose A 0.045 mg/kg/day (n = 19) |
Dose B up to 0.067 mg/kg/day (n = 15) |
Dose C up to 0.089 mg/kg/day (n = 12) |
Total (n = 46) |
|
|
Baseline height (cm)1 |
105 (12) |
108 (12.7) |
107 (11.7) |
106 (11.9) |
|
Final height (cm)1 |
157 (6.7) |
163 (6.0) |
163 (4.9) |
161 (6.5) |
|
Number (%) of patients reaching normal height (height SDS >-2 using National standard) |
10 (53%) |
12 (80%) |
10 (83%) |
32 (70%) |
|
Height SDS (Turner standard)2 | ||||
|
Final [95% CI] |
1.7 [1.4, 2.0] |
2.5 [2.1, 2.8]3 |
2.5 [2.1, 2.9]4 |
NA |
|
Change from baseline [95% CI] |
1.5 [1.2, 1.8] |
2.2 [1.9, 2.5]3 |
2.2 [1.9, 2.6]4 |
NA |
|
Height SDS (National standard)2 | ||||
|
Final [95% CI] |
-1.9 [-2.2, -1.6] |
-1.2 [-1.5, -0.9]4 |
-1.2 [-1.6, -0.8]5 |
NA |
|
Change from baseline [95% CI] |
0.7 [0.4, 1.0] |
1.4 [1.1, 1.7]4 |
1.4 [1.1, 1.8]5 |
NA |
Values are expressed as mean (SD) unless otherwise indicated. SDS: Standard deviation score.
1Unadjusted (raw) means; 2Adjusted (least squares) means based on an ANCOVA model including terms for treatment,
duration of treatment, age at baseline, bone age at baseline, height SDS at baseline, age at onset of puberty and mid-parental target height SDS;
3p=0.005 vs. Dose A; 4p=0.006 vs. Dose A; 5p=0.008 vs. Dose A
In Study 2, 19 euthyroid Caucasian patients (with bone age ≤13.9 years) were randomized to treatment with 0.067 mg/kg/day of NORDITROPINas a single subcutaneous dose in the evening, or divided into two doses (1/3 morning and 2/3 evening). All subjects were treated with concomitant ethinyl estradiol. Overall, at baseline, mean age was 13.6 years, mean height SDS (National standard) was -3.5 and mean HV during the previous year was 4.3 cm/year. Patients were treated for a mean of 3.6 years. In that there were no significant differences between the two treatment groups for any linear growth variables, the data from all patients were pooled. Overall mean final height was 155 cm in the 17 children who attained final height. Height SDS changed significantly from -3.5 at baseline to -2.4 at final height (National standard), and from 0.7 to 1.3 at final height (Turner standard).
14.4 Short Stature in Children Born Small for Gestational Age (SGA) with No Catch-up Growth by Age 2-4 Years
A multi-center, randomized, double-blind, two-arm study to final height (Study 1) and a 2-year, multi-center, randomized, double-blind, parallel-group study (Study 2) were conducted to assess the efficacy and safety of NORDITROPIN. Changes in height and height velocity were compared to a national reference population in both studies.
Study 1 included 53, 38 male, 15 female, non-GHD, Dutch prepubertal pediatric patients 3-11 years of age with short stature born SGA with no catch-up growth. Catch-up growth was defined as obtaining a height of ≥ 3rd percentile within the first 2 years of life or at a later stage. Inclusion criteria included: birth length < 3rd percentile for gestational age, and height velocity (cm/year) for chronological age < 50th percentile.Exclusion criteria included chromosomal abnormalities, signs of a syndrome (except for Silver-Russell syndrome), serious/chronic co-morbid disease, malignancy, and previous rhGH therapy. NORDITROPINwas administered subcutaneously daily at bedtime at a dose of approximately 0.033 (Dose A) or 0.067 mg/kg/day (Dose B) for the entire treatment period. Final height was defined as a height velocity below 2 cm/year. Treatment with NORDITROPINwas continued to final height for up to 13 years. Mean duration of treatment was 9.5 years (boys) and 7.9 years (girls).
38 out of 53 children (72%) reached final height. Sixty-three percent (24 out of 38) of the children who reached final height were within the normal range of their healthy peers (Dutch national reference). For both doses combined, actual mean final height was 171 (SD 6.1) cm in boys and 159 (SD 4.3) cm in girls.
As seen in Table 6, for boys and girls combined, both mean final height SDS, and increase in height SDS from baseline to final height, were significantly greater after treatment with Dose B (0.067 mg/kg/day). A similar dose response was observed for the increase in height SDS from baseline to Year 2 (Table 6).
Overall mean height velocity at baseline was 5.4 cm/y (SD 1.2; n=29). Height velocity was greatest during the first year of NORDITROPINtreatment and was significantly greater after treatment with Dose B (mean 11.1 cm/y [SD 1.9; n=19]) compared with Dose A (mean 9.7 cm/y [SD 1.3; n=10]).
Table 6 – Study 1: Results for Final Height SDS and Change from Baseline to Final Height in Height SDS Using National Standard After Long-Term Treatment of SGA Children with NORDITROPIN
|
Raw Mean ± SD (N) |
|||
|
Dose A 0.033 mg/kg/day |
Dose B 0.067 mg/kg/day |
Total |
|
|
Baseline Height SDS |
-3.2 ± 0.7 (26) |
-3.2 ± 0.7 (27) |
-3.2 ± 0.7 (53) |
|
Adjusted least-squares mean ± standard error (N), Treatment Difference [95% confidence intervals] |
|||
|
Height SDS: Change from Baseline at Year 22 |
1.4 ± 0.1 (26) |
1.8 ± 0.1 (26) |
Treatment Diff = 0.4 [0.2, 0.7]3 |
|
Height SDS: Change from Baseline at Final Height1 |
1.4 ± 0.2 (19) |
1.8 ± 0.2 (19) |
Treatment Diff = 0.5 [0.0, 0.9]3 |
|
Final Height SDS1 |
-1.8 ± 0.2 (19) |
-1.3 ± 0.2 (19) |
|
|
Final Height SDS > -2 |
13/19 (68%) |
11/19 (58%) |
24/38 (63%) |
SDS: Standard deviation score.
1Adjusted (least-squares) means based on an ANCOVA model including terms for treatment, gender, age at baseline, bone age at baseline, height SDS at baseline, duration of treatment, peak GH after stimulation and baseline IGF-1.
2 Adjusted (least-squares) means based on an ANCOVA model including terms for treatment, gender, age at baseline, height SDS at baseline, and pubertal status.
3 p<0.05
In study 2, 84 randomized, prepubertal, non-GHD, Japanese children (age 3-8) were treated for 2 years with 0.033 or 0.067 mg/kg/day of NORDITROPIN subcutaneously daily at bedtime or received no treatment for 1 year. Additional inclusion criteria included birth length or weight SDS ≤ -2 or < 10th percentile for gestational age, height SDS for chronological age ≤ -2, and height velocity SDS for chronological age < 0 within one year prior to Visit 1. Exclusion criteria included diabetes mellitus, history or presence of active malignancy, and serious co-morbid conditions.
As seen in Table 7, for boys and girls combined, there was a dose-dependent increase in height SDS at Year 1 and Year 2. The increase in height SDS from baseline to Year 2 (0.033 mg/kg/day, 0.8 vs. 0.067 mg/kg/day, 1.4) was significantly greater after treatment with 0.067 mg/kg/day. In addition, the increase in height SDS at Year 1 was significantly greater in both active treatment groups compared to the untreated control group.
Table 7 – Study 2: Results for Change from Baseline in Height SDS At Year 1 and Year 2 Using National Standard After Short-Term Treatment of SGA Children with NORDITROPIN
|
Raw Mean ± SD (N) |
|||
|
No Treatment |
0.033 mg/kg/day |
0.067 mg/kg/day |
|
|
Height SDS: Baseline |
-2.9 ± 0.5 (15) |
-3.0 ± 0.6 (35) |
-2.9 ± 0.7 (34) |
|
Height SDS: Year 1 |
-2.8 ± 0.5 (15) |
-2.4 ± 0.6 (33) |
-2.0 ± 0.8 (34) |
|
Height SDS: Year 2 |
NA |
-2.2 ± 0.7 (33) |
-1.4 ± 0.7 (32) |
|
Adjusted least-squares mean ± standard error (N), Treatment Diff [95% confidence intervals] |
|||
|
Height SDS: Change from Baseline at Year 11 |
0.1 ± 0.1 (15) |
0.6 ± 0.1 (33) |
0.9 ± 0.1 (34) |
|
0.033 vs. No Treatment: Treatment Diff = 0.5, [0.3, 0.7]2 0.067 vs. No Treatment: Treatment Diff = 0.8, [0.6, 1.0]2 0.067 vs. 0.033: Treatment Diff = 0.3, [0.2, 0.5]2 |
|||
|
Height SDS: Change from Baseline at Year 21 |
NA |
0.8 ± 0.1 (33) |
1.4 ± 0.1 (32) |
|
0.067 vs. 0.033: Treatment Diff = 0.6, [0.5, 0.8], p-value < 0.0001 |
|||
SDS: Standard deviation score.
1Adjusted (least-squares) means based on an ANCOVA model including terms for treatment, gender, age at baseline, and height SDS at baseline. All children remained prepubertal during the study.
2 p< 0.0001
14.5 Idiopathic Short Stature (ISS)
The efficacy and safety of another somatropin product was evaluated in 105 patients who were retrospectively identified as having ISS in a randomized, open-label, clinical study. Patients were enrolled on the basis of short stature, stimulated GH secretion > 10 ng/mL, and prepubertal status. All patients were observed for height progression for 12 months and were subsequently randomized to this other somatropin product or observation only and followed to final height. Two doses of this other somatropin product were evaluated in this trial: 0.23 mg/kg/week (0.033 mg/kg/day) and 0.47 mg/kg/week (0.067 mg/kg/day). Baseline patient characteristics for the ISS patients who remained prepubertal at randomization (n= 105) were: mean (± SD): chronological age 11.4 (1.3) years, height SDS -2.4 (0.4), height velocity SDS -1.1 (0.8), and height velocity 4.4 (0.9) cm/yr, IGF-1 SDS -0.8 (1.4). Patients were treated for a median duration of 5.7 years. Results for final height SDS are displayed by treatment arm in Table 8. The observed mean gain in final height was 9.8 cm for females and 5.0 cm for males for both doses combined compared to untreated control subjects. A height gain of 1 SDS was observed in 10% of untreated subjects, 50% of subjects receiving 0.23 mg/kg/week and 69% of subjects receiving 0.47 mg/kg/week.
Table 8 – Final height SDS results for pre-pubertal patients with ISS*
|
|
Another Somatropin Product |
||||
|
Untreated (n=30) |
0.033 mg/kg/day (n=30) |
0.067 mg/kg/day (n=42) |
0.033 vs Untreated (95% CI) |
0.067 vs Untreated (95% CI) |
|
|
Baseline height SDS Final height SDS minus baseline |
0.41 (0.58) |
0.95 (0.75) |
1.36 (0.64) |
+0.53 (0.20, 0.87)** |
+0.94 (0.63, 1.26)** |
|
Baseline predicted ht Final height SDS minus baseline predicted final height SDS |
0.23 (0.66) |
0.73 (0.63) |
1.05 (0.83) |
+0.60 (0.09, 1.11)** |
+0.90 (0.42, 1.39)** |
Least square means based on ANCOVA (final height SDS and final height SDS minus baseline predicted height SDS were adjusted for baseline height SDS)
* Mean (SD) are observed values
**p<0.05
14.6 Growth Failure Due to Prader-Willi Syndrome (PWS)
The safety and efficacy of another somatropin product were evaluated in two randomized, open-label, controlled clinical studies. Patients received either this other somatropin product or no treatment for the first year of the studies, while all patients received this other somatropin product during the second year. This other somatropin product was administered as a daily SC injection, and the dose was calculated for each patient every 3 months. In Study 1, the treatment group received this other somatropin product at a dose of 0.24 mg/kg/week during the entire study. During the second year, the control group received this other somatropin product at a dose of 0.48 mg/kg/week. In Study 2, the treatment group received this other somatropin product at a dose of 0.36 mg/kg/week during the entire study. During the second year, the control group received this other somatropin product at a dose of 0.36 mg/kg/week.
The results are presented in Table 9. Linear growth continued to increase in the second year, when both groups received treatment with this other somatropin product.
|
Study 1 |
Study 2 |
|||
|
Another Somatropin Product (0.24 mg/kg/week) (n=15) |
Untreated Control (n=12) |
Another Somatropin Product (0.36 mg/kg/week) (n=7) |
Untreated Control (n=9) |
|
|
Linear growth (cm) Baseline height |
112.7 ± 14.9 |
109.5 ± 12.0 |
120.3 ± 17.5 |
120.5 ± 11.2 |
|
Growth from 0 to 12 months |
11.6* ± 2.3 |
5.0 ± 1.2 |
10.7* ± 2.3 |
4.3 ± 1.5 |
|
Baseline SDS |
-1.6 ± 1.3 |
-1.8 ± 1.5 |
-2.6 ± 1.7 |
-2.1 ± 1.4 |
|
SDS at 12 months |
-0.5* ± 1.3 |
-1.9 ± 1.4 |
-1.4* ± 1.5 |
-2.2 ± 1.4 |
* p <0.05
14.7 Adults with Growth Hormone Deficiency (GHD)
A total of six randomized, double-blind, placebo-controlled studies were performed. Two representative studies, one in adult onset (AO) GHD patients and a second in childhood onset (CO) GHD patients, are described below.
Study 1
A single center, randomized, double-blind, placebo-controlled, parallel-group, six month clinical trial was conducted in 31 adults with AO GHD comparing the effects of NORDITROPIN(somatropin) injection and placebo on body composition. Patients in the active treatment arm were treated with NORDITROPIN0.017 mg/kg/day (not to exceed 1.33 mg/day). The changes from baseline in lean body mass (LBM) and percent total body fat (TBF) were measured by total body potassium (TBP) after 6 months.
Treatment with NORDITROPINproduced a significant increase from baseline in LBM compared to placebo (Table 10).
Table 10 – Lean Body Mass (kg) by TBP
|
NORDITROPIN (n=15) |
Placebo (n=16) |
|
|
Baseline (mean) |
50.27 |
51.72 |
|
Change from baseline at 6 months (mean) |
1.12 |
-0.63 |
|
Treatment difference (mean) 95% confidence interval p-value |
1.74 (0.65, 2.83) p=0.0028* |
|
*Least square mean based on an ANOVA model including treatment and sex as factors
Analysis of the treatment difference on the change from baseline in percent TBF revealed a significant decrease in the NORDITROPIN-treated group compared to the placebo group (Table 11).
Table 11 – Total Body Fat (%) by TBP
|
NORDITROPIN (n=15) |
Placebo (n=16) |
|
|
Baseline (mean) |
44.74 |
42.26 |
|
Change from baseline at 6 months (mean) |
-2.83 |
1.92 |
|
Treatment difference (mean) 95% confidence interval p-value |
-4.74 (-7.18, -2.30) p=0.0004* |
|
*Least square mean based on an ANOVA model including treatment and sex as factors
NORDITROPINalso significantly increased serum osteocalcin (a marker of osteoblastic activity).
Study 2
A single center, randomized, double-blind, placebo-controlled, parallel-group, dose-finding, six month clinical trial was conducted in 49 men with CO GHD comparing the effects of NORDITROPINand placebo on body composition. Patients were randomized to placebo or one of three active treatment groups (0.008, 0.016, and 0.024 mg/kg/day). Thirty three percent of the total dose to which each patient was randomized was administered during weeks 1-4, 67% during weeks 5-8, and 100% for the remainder of the study. The changes from baseline in LBM and percent TBF were measured by TBP after 6 months.
Treatment with NORDITROPINproduced a significant increase from baseline in LBM compared to placebo (pooled data) (Table 12).
Table 12 – Lean Body Mass (kg) by TBP
|
NORDITROPIN (n=36) |
Placebo (n=13) |
|
|
Baseline (mean) |
48.18 |
48.90 |
|
Change from baseline at 6 months (mean) |
2.06 |
0.70 |
|
Treatment difference (mean) 95% confidence interval p-value |
1.40 (0.39, 2.41) p=0.0079* |
|
*Least square mean based on an ANOVA model including treatment as a factor
Analysis of the treatment difference on the change from baseline in percent TBF revealed a significant decrease in the NORDITROPIN-treated groups (pooled data) compared to the placebo group (Table 13).
Table 13 – Total Body Fat (%) by TBP
|
NORDITROPIN (n=36) |
Placebo (n=13) |
|
|
Baseline (mean) |
34.55 |
34.07 |
|
Change from baseline at 6 months (mean) |
-6.00 |
-1.78 |
|
Treatment difference (mean) 95% confidence interval p-value |
-4.24 (-7.11, -1.37) p=0.0048* |
|
*Least square mean based on an ANOVA model including treatment as a factor
16 HOW SUPPLIED/STORAGE AND HANDLING
NORDITROPINinjection is a clear and colorless solution available as FlexPro prefilled pens:
- •
- NORDITROPINFlexPro 5 mg/1.5 mL (orange) NDC 0169-7704-21
- •
- NORDITROPIN FlexPro 10 mg/1.5 mL (blue) NDC 0169-7705-21
- •
- NORDITROPIN FlexPro 15 mg/1.5 mL (green) NDC 0169-7708-21
- •
- NORDITROPIN FlexPro 30 mg/3 mL (purple) NDC 0169-7703-21
Each NORDITROPIN FlexPro pen is for use by a single patient. A NORDITROPIN FlexPro pen must never be shared between patients, even if the needle is changed.
Unused NORDITROPINFlexPro prefilled pensmust be stored at 2°C to 8°C/36°F to 46°F (refrigerator). Do not freeze. Avoid direct light.
Table 14 – Storage Conditions and Expiration
|
Before Use |
In-use (After 1st injection) |
|
|
Storage requirement |
Storage Option 1 (Refrigeration) |
Storage Option 2 (Room temperature) |
|
2ºC to 8 ºC/ 36ºF to 46 ºF Until exp. date |
2ºC to 8 ºC/36ºF to 46 ºF 4 weeks |
Up to 25ºC/77ºF 3 weeks |
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling.
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Neoplasms – Advise childhood cancer survivors/caregivers that individuals treated with brain/head radiation are at increased risk of secondary neoplasms and as a precaution need to be monitored for recurrence. Advise patients/caregivers to report marked changes in behavior, onset of headaches, vision disturbances and/or changes in skin pigmentation or changes in the appearance of pre-existing nevi.
- •
- Fluid Retention - Advise patients that fluid retention during NORDITROPINreplacement therapy in adults may frequently occur. Inform patients of the clinical manifestations of fluid retention (e.g. edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) and to report to their healthcare provider any of these signs or symptoms occur during treatment with NORDITROPIN.
- •
- Pancreatitis - Advise patients/caregivers that pancreatitis may develop and to report to their healthcare provider any new onset abdominal pain.
- •
- Hypoadrenalism - Advise patients/caregivers who have or who are at risk for pituitary hormone deficiency(s) that hypoadrenalism may develop and to report to their healthcare provider if they experience hyperpigmentation, extreme fatigue, dizziness, weakness, or weight loss.
- •
- Hypothyroidism - Advise patients/caregivers that undiagnosed/untreated hypothyroidism may prevent an optimal response to NORDITROPIN. Advise patients/caregivers they may require periodic thyroid function tests.
- •
- Intracranial Hypertension - Advise patients/caregivers to report to their healthcare provider any visual changes, headache, and nausea and/or vomiting.
- •
- Hypersensitivity Reactions – Advise patients/caregivers that serious systemic hypersensitivity reactions (anaphylaxis and angioedema) are possible and that prompt medical attention should be sought if an allergic reaction occurs.
- •
- Glucose Intolerance/ Diabetes Mellitus – Advise patients/caregivers that new onset impaired glucose intolerance/diabetes mellitus or exacerbation of preexisting diabetes mellitus can occur and monitoring of blood glucose during treatment with NORDITROPINmay be needed.
Novo Nordisk® is a registered trademark of Novo Nordisk A/S.
NORDITROPIN® and FlexPro® are registered trademarks of Novo Nordisk Health Care AG.
© 2002-2020 Novo Nordisk Health Care AG
For information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536, USA
1-888-668-6444
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
PATIENT INFORMATION
NORDITROPIN® (Nor-dee-tro-pin)
(somatropin) injection for subcutaneous use
What is NORDITROPIN?
NORDITROPIN is a prescription medicine that contains human growth hormone, the same growth hormone made by the human body.
NORDITROPIN is given by injection under the skin (subcutaneous) and is used to treat:
- •
- children who are not growing because of low or no growth hormone.
- •
- children who are short (in stature) and who have Noonan syndrome, Turner syndrome, or were born small (small for gestational age-SGA) and have not caught-up in growth by age 2 to 4 years.
- •
- children who have Idiopathic Short Stature (ISS).
- •
- children who are not growing who have Prader-Willi syndrome (PWS).
- •
- adults who do not make enough growth hormone.
Do not use NORDITROPIN if:
- •
- you have a critical illness caused by certain types of heart or stomach surgery, trauma or breathing (respiratory) problems.
- •
- you are a child with Prader-Willi syndrome who is severely obese or has breathing problems including sleep apnea (briefly stop breathing during sleep).
- •
- you have cancer or other tumors.
- •
- you are allergic to somatropin or any of the ingredients in NORDITROPIN. See the end of this leaflet for a complete list of ingredients in NORDITROPIN.
- •
- your healthcare provider tells you that you have certain types of eye problems caused by diabetes (diabetic retinopathy).
- •
- you are a child with closed bone growth plates (epiphyses).
Before taking NORDITROPIN, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have had heart or stomach surgery, trauma or serious breathing (respiratory) problems.
- •
- have had a history of problems breathing while you sleep (sleep apnea).
- •
- have or have had cancer or any tumor.
- •
- have diabetes.
- •
- are pregnant or plan to become pregnant. It is not known if NORDITROPIN will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- •
- are breastfeeding or plan to breastfeed. It is not known if NORDITROPIN passes into your breast milk. You and your healthcare provider should decide if you will take NORDITROPIN while you breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. NORDITROPIN may affect how other medicines work, and other medicines may affect how NORDITROPIN works.
How should I use NORDITROPIN?
- •
- Read the detailed Instructions for Use that come with NORDITROPIN.
- •
- NORDITROPIN comes in 4 different dosage strengths. Your healthcare provider will prescribe the dose that is right for you.
- •
- Your healthcare provider will show you how to inject NORDITROPIN.
- •
- Use NORDITROPIN exactly as your healthcare provider tells you to.
- •
- NORDITROPIN FlexPro pens are for use by 1 person only.
- •
- Do not share your NORDITROPIN pens and needles with another person, even if the needle has been changed. You may give another person an infection or get an infection from them.
What are the possible side effects of NORDITROPIN?
NORDITROPIN may cause serious side effects, including:
- •
- high risk of death in people who have critical illnesses because of heart or stomach surgery, trauma or serious breathing (respiratory) problems.
- •
- high risk of sudden death in children with Prader-Willi syndrome who are severely obese or have breathing problems, including sleep apnea.
- •
- increased risk of growth of cancer or a tumor that is already present and increased risk of the return of cancer or a tumor in people who were treated with radiation to the brain or head as children and who developed low growth hormone problems. Your or your child’s healthcare provider will need to monitor you or your child for a return of cancer or a tumor. Contact the healthcare provider if you or your child starts to have headaches, or have changes in behavior, changes in vision, or changes in moles, birthmarks, or the color of your skin.
- •
- new or worsening high blood sugar (hyperglycemia) or diabetes. Your or your child’s blood sugar may need to be monitored during treatment with NORDITROPIN.
- •
- increase in pressure in the skull (intracranial hypertension). If you or your child has headaches, eye problems, nausea or vomiting, contact the healthcare provider.
- •
- serious allergic reactions. Get medical help right away if you or your child has the following symptoms:
- o
- swelling of your face, lips, mouth, or tongue
- o
- trouble breathing
- o
- wheezing
- o
- severe itching
- o
- skin rashes, redness, or swelling
- o
- dizziness or fainting
- o
- fast heartbeat or pounding in your chest
- o
- sweating
- •
- your body holding too much fluid (fluid retention) such as swelling in the hands and feet, pain in your joints or muscles or nerve problems that cause pain, burning or tingling in the hands, arms, legs and feet. Fluid retention can happen in adults during treatment with NORDITROPIN. Tell your healthcare provider if you have any of these signs or symptoms of fluid retention.
- •
- decrease in a hormone called cortisol. The healthcare provider will do blood tests to check your or your child’s cortisol levels. Tell your or your child’s healthcare provider if you or your child has darkening of the skin, severe fatigue, dizziness, weakness, or weight loss.
- •
- decrease in thyroid hormone levels. Decreased thyroid hormone levels may affect how well NORDITROPIN works. The healthcare provider will do blood tests to check your or your child’s thyroid hormone levels.
- •
- hip and knee pain or a limp in children (slipped capital femoral epiphysis)
- •
- worsening of curvature of the spine (scoliosis)
- •
- severe and constant abdominal pain. This could be a sign of pancreatitis. Tell your or your child’s healthcare provider if you or your child has any new abdominal pain.
- •
- loss of fat and tissue weakness in the area of skin you inject. Talk to your healthcare provider about rotating the areas where you inject NORDITROPIN.
- •
- increase in phosphorus, alkaline phosphatase and parathyroid hormone levels in your blood. Your or your child’s healthcare provider will do blood tests to check this.
The most common side effects of NORDITROPIN include:
- •
- injection site reactions and rashes
- •
- headaches
These are not all the possible side effects of NORDITROPIN.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Novo Nordisk at 1-888-668-6444.
How should I store NORDITROPIN?
- •
-
Before you use NORDITROPIN FlexPro pens for the first time:
- o
- Store your new, unused NORDITROPIN pen in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- o
- Do not freeze NORDITROPIN.
- o
- Keep NORDITROPIN away from direct light.
- o
- Do not use NORDITROPIN that has been frozen or in temperatures warmer than 77ºF (25ºC).
- o
- Do not use NORDITROPIN after the expiration date printed on the carton and the pen.
- •
-
After you use NORDITROPIN FlexPro pens and there is still medicine left:
- o
- Store remaining NORDITROPIN in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC) and use within 4 weeks, or
- o
- Store remaining NORDITROPIN at room temperature no warmer than 77ºF (25ºC) and use within 3 weeks.
Keep NORDITROPIN and all medicines out of the reach of children.
General information about the safe and effective use of NORDITROPIN.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NORDITROPIN for a condition for which it was not prescribed. Do not give NORDITROPIN to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NORDITROPIN that is written for health professionals.
What are the ingredients in NORDITROPIN?
Active ingredient: somatropin
Inactive ingredients: Histidine, Poloxamer 188, Phenol, Mannitol, HCl/NaOH (as needed) and Water for Injection
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
This Patient Information has been approved by the U.S.Food and Drug Administration.
Revised: 02/2018
INSTRUCTIONS FOR USE
Norditropin® (Nor-dee-tro-pin) FlexPro®
(somatropin) injection
5 mg/1.5 mL
Norditropin FlexPro Pen is for single-patient-use only.
Supplies you will need:
- •
- Norditropin FlexPro prefilled Pen new injection needle. Norditropin prefilled Pen is designed to be used with all Novo Nordisk disposable needles up to a length of 8 mm
- •
- sharps disposal container. See step 5 for information on how to throw away (dispose of) used needles and Pens.
- •
- alcohol pad
- •
- gauze pad
How to use your Norditropin FlexPro Pen
5 steps you should follow for a Norditropin injection:
- Step 1: Prepare your Norditropin FlexPro Pen
- Step 2: Check the Norditropin flow with each new Pen
- Step 3: Select your dose
- Step 4: Inject your dose
- Step 5: After your injection
For further information about your Pen see:
- Frequently Asked Questions
- Important information
- Patient Information
Make sure that you read this information carefully.
Norditropin is for use under the skin only (subcutaneous).
Do not share your Norditropin Pen and needles with another person. You may give another person an infection or get an infection from them.
Do not use your Pen without proper training from your healthcare provider.
Make sure that you are confident in giving an injection with the Pen before you start your treatment.
If you are blind or have poor eyesight and cannot read the dose counter on the Pen, do not use this Pen without help. Get help from a person with good eyesight who is trained to use the Pen.
Step 1. Prepare your Norditropin FlexPro Pen
- •
- Wash your hands with soap and water.
- •
- Check the name, strength, and colored label on your Pen to make sure that it contains Norditropin in the right strength.
- •
- Pull off the Pen cap.
- •
- Turn the Pen upside down 1 or 2 times to check that the Norditropin in your Pen is clear and colorless.
- See figure A. If the Norditropin looks cloudy, do not use the Pen.
- •
- When you are ready to give your injection, take a new disposable needle, and remove the paper tab.
- •
- Push the needle straight onto the Pen. Turn the needle clockwise until it is on tight. See figure B.
 Always use a new needle for each injection. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
Always use a new needle for each injection. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
- •
- Pull off the outer needle cap and dispose of it. See figure C.
- •
- Pull off the inner needle cap and dispose of it. See figure D.
 A drop of Norditropin may appear at the needle tip. This is normal, but you must still check the Norditropin flow with each new Pen. See step 2.
A drop of Norditropin may appear at the needle tip. This is normal, but you must still check the Norditropin flow with each new Pen. See step 2.
 Never use a bent or damaged needle.
Never use a bent or damaged needle.
Step 2. Check the Norditropin flow with each new Pen
 If your Pen is already in use, go to step 3.
If your Pen is already in use, go to step 3.
- Before using a new Pen, check the Norditropin flow to make sure the growth hormone can flow through the Pen and needle.
- •
- Turn the dose selector clockwise 1 tick marking on the dose counter to select 0.025 mg. You will hear a faint “click” when you turn the dose selector. See figure E.
- •
- 1 marking on the dose counter equals 0.025 mg. See figure F.
- •
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter returns to “0”. The “0” must line up with the dose pointer. See figure G.
- •
- Check that a drop of Norditropin appears at the needle tip. See figure H.
 If no Norditropin appears, repeat step 2 up to 6 times.
If no Norditropin appears, repeat step 2 up to 6 times.
- If you still do not see a drop of Norditropin, change the needle:
- •
- Carefully remove the needle from the Pen by turning the needle counterclockwise. Place the needle in a sharps disposal container immediately. See step 5.
- •
- and repeat step 2 again.
- Do not use the Pen if a drop of Norditropin still does not appear after changing the needle and repeating step 2. Call Novo Nordisk at
- 1-888-668-6444 for help.
Step 3. Select your dose
- •
- To start, check that the dose pointer is set at “0”.
- •
- Turn the dose selector clockwise to select the dose you need. See figure I.
When you have selected your dose, you can go to step 4.
 If there is not enough Norditropin left to select a full dose, see Frequently Asked Questions.
If there is not enough Norditropin left to select a full dose, see Frequently Asked Questions.
 The dose counter shows the dose in “mg”. See figures J and K. Always use the dose counter to select the exact dose. Do not use the “click” sounds you hear when you turn the dose selector or the Pen scale to select your dose. Only the dose pointer on the dose counter will show the exact dose selected.
The dose counter shows the dose in “mg”. See figures J and K. Always use the dose counter to select the exact dose. Do not use the “click” sounds you hear when you turn the dose selector or the Pen scale to select your dose. Only the dose pointer on the dose counter will show the exact dose selected.
 If you select the wrong dose, you can turn the dose selector clockwise or counterclockwise to the correct dose. See figure L.
If you select the wrong dose, you can turn the dose selector clockwise or counterclockwise to the correct dose. See figure L.
- The Pen “clicks” sound and feel differently when the dose selector is turned clockwise, counterclockwise, or if you forcefully move it past the number of “mg” left in the Pen.
Step 4. Inject your dose
- •
- Select the injection site.
- •
- Norditropin can be injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs (thighs), or upper arms, as instructed by your healthcare provider. Change the injection site every day.
- •
- Wipe the injection site with an alcohol swab and let the area dry.
- •
- Insert the needle into your skin as your healthcare provider has shown you. See figure M.
- Make sure you can see the dose counter. Do not cover it with your fingers. This could block the injection.
- •
- Press and hold down the dose button until the dose counter shows “0”. See figure N. The “0” must line up with the dose pointer. You may then hear or feel a “click”.
- •
- Continue to hold the needle in your skin.
 If “0” does not appear in the dose counter after continuously pressing the dose button, your needle may be blocked or damaged, see Frequently Asked Questions.
If “0” does not appear in the dose counter after continuously pressing the dose button, your needle may be blocked or damaged, see Frequently Asked Questions.
- •
- Keep the needle in your skin after the dose counter has returned to “0”. Count slowly to 6 to ensure that the full dose has been delivered. See figure O.
- •
- Carefully remove the needle from your skin. See figure P. If blood appears at the injection site, press lightly with a gauze pad. Do not rub the area.
 You may see a drop of Norditropin at the needle tip after injecting. This is normal and does not affect your dose.
You may see a drop of Norditropin at the needle tip after injecting. This is normal and does not affect your dose.
Step 5. After your injection
- •
- Carefully remove the needle from the Pen by turning the needle counterclockwise. See figure Q.
- •
- Place the needle in a sharps disposal container immediately to reduce the risk of a needle stick. See figure R.
 Always dispose of the needle after each injection.
Always dispose of the needle after each injection.
- For further information about safe sharps disposal, see Frequently Asked Questions.
- Do not try to put the needle cap back on . You may stick yourself with the needle.
- •
- Put the Pen cap on your Pen after each use to protect the Norditropin from direct light. See figure S.
- See “How should I store Norditropin?”.
- Always remove the needle from your Pen. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
- How should I store Norditropin?
- •
- Before you use Norditropin FlexPro pens for the first time:
- o
- Store your new, unused Norditropin pen in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- o
- Do not freeze Norditropin.
- o
- Keep Norditropin away from direct light.
- o
- Do not use Norditropin that has been frozen or in temperatures warmer than 77ºF (25ºC).
- o
- Do not use Norditropin after the expiration date printed on the carton and the pen.
- •
- After you use Norditropin FlexPro pens and there is still medicine left:
- o
- Store remaining Norditropin in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC) and use within 4 weeks, or
- o
- Store remaining Norditropin at room temperature no warmer than 77ºF (25ºC) and use within 3 weeks.
Keep Norditropin and all medicines out of the reach of children.
Frequently Asked Questions
How do I see how much Norditropin is left in my Pen?
The Pen scale shows you approximately how much Norditropin is left in your Pen. See figure T below.
To see how much Norditropin is left in your Pen, use the dose counter: Turn the dose selector clockwise until the dose counter stops. The dose pointer will line up with the number of “mg” left in the Pen. You can select a maximum dose of 2.0 mg. If the dose counter stops with the dose pointer lined up with “2.0” , at least 2.0 mg are left in your Pen.
If the dose counter stops with the dose pointer lined up with “1.25”, only 1.25 mg are left in your Pen. See figure U below.
What if I need a larger dose than what is left in my Pen?
It is not possible to select a larger dose on the dose counter than the number of “mg” left in your Pen.
If you need more Norditropin than you have left in your Pen, you can use a new Pen or split your dose between your current Pen and a new Pen. Only split your dose if you have been trained or advised by your healthcare provider on how to do this. You may find it helpful to use a calculator to plan the doses as instructed by your healthcare provider.
Be very careful to calculate your split dose correctly so that you do not give the wrong dose. If you are not sure how to split your dose using two Pens, then select and inject the dose you need with a new Pen.
What if no Norditropin appears when I check the flow?
A. Your needle may be blocked or damaged, if no Norditropin appears at the needle tip. Remove the needle as described in step 5 and repeat steps 1 and 2.
B. Your Pen may be defective, if Norditropin still does not appear after changing the needle. Do not use the Pen. Contact Novo Nordisk at 1-888-668-6444.
What if “0” does not appear after completing my injection?
The needle may be blocked or damaged, and you have not received any Norditropin – even though the dose counter has moved from the dose that you have set. Remove the needle as described in step 5 and repeat steps 1 to 4.
If “0” still does not appear after completing the injection, contact Novo Nordisk at 1-888-668-6444.
How should I take care of my Pen?
Be careful not to drop your Pen or knock it against hard surfaces. Do not expose your Pen to dust, dirt, liquid, or direct light.
See “How should I store Norditropin?”.
Do not try to refill your Pen, it is already prefilled. When your Pen is empty, throw it away and use a new pen. See “How do I dispose of used needles and Pens?”.
Frequently Asked Questions
What if I drop my Pen?
If you drop your Pen or think that something is wrong with it, attach a new disposable needle and check the Norditropin flow before you inject, see steps 1 and 2. Do not try to repair your Pen or pull it apart.
How do I clean my Pen?
Do not wash, soak, or lubricate your Pen. If necessary, clean it with mild detergent on a moistened cloth.
How do I dispose of used needles and Pens?
Put your used needles in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- •
- made of a heavy-duty plastic,
- •
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- •
- upright and stable during use,
- •
- leak-resistant, and
- •
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should dispose of used needles and Pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
When there is not enough medicine left in your Pen for your prescribed dose, the Pen may be thrown away in your household trash after you have removed the needle.
- •
- Caregivers must be very careful when handling needles to reduce the risk of needle sticks and infection.
- •
- Norditropin® FlexPro® 5 mg/1.5 mL Pen is compatible with FlexPro® PenMate®.
www.norditropin.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
PATENT Information: http://novonordisk-us.com/patients/products/product-patents.html
Norditropin® and FlexPro® are registered trademarks of Novo Nordisk Health Care AG.
Novo Nordisk® and PenMate®are registered trademarks of Novo Nordisk A/S.
© 2004-2020 Novo Nordisk Health Care AG
For further information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-888-668-6444
norditropin-us.com
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark
Revised 3/2020
INSTRUCTIONS FOR USE
Norditropin® (Nor-dee-tro-pin) FlexPro®
(somatropin) injection
10 mg/1.5 mL
Norditropin FlexPro Pen is for single-patient-use only.
Supplies you will need:
- •
- Norditropin FlexPro prefilled Pen new injection needle. Norditropin prefilled Pen is designed to be used with all Novo Nordisk disposable needles up to a length of 8 mm.
- •
- sharps disposal container. See step 5 for information on how to throw away (dispose of) used needles and Pens.
- •
- alcohol pad
- •
- gauze pad
How to use your Norditropin FlexPro Pen
5 steps you should follow for a Norditropin injection:
- Step 1: Prepare your Norditropin FlexPro Pen
- Step 2: Check the Norditropin flow with each new Pen
- Step 3: Select your dose
- Step 4: Inject your dose
- Step 5: After your injection
For further information about your Pen see:
- Frequently Asked Questions
- Important information
- Patient Information
Make sure that you read this information carefully.
Norditropin is for use under the skin only (subcutaneous).
Do not share your Norditropin Pen and needles with another person. You may give another person an infection or get an infection from them.
Do not use your Pen without proper training from your healthcare provider.
Make sure that you are confident in giving an injection with the Pen before you start your treatment.
If you are blind or have poor eyesight and cannot read the dose counter on the Pen, do not use this Pen without help. Get help from a person with good eyesight who is trained to use the Pen.
Step 1. Prepare your Norditropin FlexPro Pen
- •
- Wash your hands with soap and water.
- •
- Check the name, strength, and colored label on your Pen to make sure that it contains Norditropin in the right strength.
- •
- Pull off the Pen cap.
- •
- Turn the Pen upside down 1 or 2 times to check that the Norditropin in your Pen is clear and colorless.
- See figure A. If the Norditropin looks cloudy, do not use the Pen.
- •
- When you are ready to give your injection, take a new disposable needle, and remove the paper tab.
- •
- Push the needle straight onto the Pen. Turn the needle clockwise until it is on tight. See figure B.
- Always use a new needle for each injection. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
- •
- Pull off the outer needle cap and dispose of it. See figure C.
- •
- Pull off the inner needle cap and dispose of it. See figure D.
 A drop of Norditropin may appear at the needle tip. This is normal, but you must still check the Norditropin flow with each new Pen. See step 2.
A drop of Norditropin may appear at the needle tip. This is normal, but you must still check the Norditropin flow with each new Pen. See step 2.
- Never use a bent or damaged needle.
Step 2. Check the Norditropin flow with each new Pen
 If your Pen is already in use, go to step 3.
If your Pen is already in use, go to step 3.
- Before using a new Pen, check the Norditropin flow to make sure the growth hormone can flow through the Pen and needle.
- •
- Turn the dose selector clockwise 1 tick marking on the dose counter to select 0.05 mg. You will hear a faint “click” when you turn the dose selector. See figure E.
- •
- 1 marking on the dose counter equals 0.05 mg. See figure F.
- •
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter returns to “0”. The “0” must line up with the dose pointer. See figure G.
- •
- Check that a drop of Norditropin appears at the needle tip. See figure H.
 If no Norditropin appears, repeat step 2 up to 6 times.
If no Norditropin appears, repeat step 2 up to 6 times.
If you still do not see a drop of Norditropin, change the needle:
- •
- Carefully remove the needle from the Pen by turning the needle counterclockwise. Place the needle in a sharps disposal container immediately. See step 5.
- •
- and repeat step 2 again.
- Do not use the Pen if a drop of Norditropin still does not appear after changing the needle and repeating step 2. Call Novo Nordisk at
- 1-888-668-6444 for help.
Step 3. Select your dose
- •
- To start, check that the dose pointer is set at “0”.
- •
- Turn the dose selector clockwise to select the dose you need. See figure I.
When you have selected your dose, you can go to step 4.
 If there is not enough Norditropin left to select a full dose, see Frequently Asked Questions.
If there is not enough Norditropin left to select a full dose, see Frequently Asked Questions.
 The dose counter shows the dose in “mg”. See figures J and K. Always use the dose counter to select the exact dose. Do not use the “click” sounds you hear when you turn the dose selector or the Pen scale to select your dose. Only the dose pointer on the dose counter will show the exact dose selected.
The dose counter shows the dose in “mg”. See figures J and K. Always use the dose counter to select the exact dose. Do not use the “click” sounds you hear when you turn the dose selector or the Pen scale to select your dose. Only the dose pointer on the dose counter will show the exact dose selected.
- If you select the wrong dose, you can turn the dose selector clockwise or counterclockwise to the correct dose. See figure L.
- The Pen “clicks” sound and feel differently when the dose selector is turned clockwise, counterclockwise, or if you forcefully move it past the number of “mg” left in the Pen.
Step 4. Inject your dose
- •
- Select the injection site.
- •
- Norditropin can be injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs (thighs), or upper arms, as instructed by your healthcare provider. Change the injection site every day.
- •
- Wipe the injection site with an alcohol swab and let the area dry.
- •
- Insert the needle into your skin as your healthcare provider has shown you. See figure M.
- Make sure you can see the dose counter. Do not cover it with your fingers. This could block the injection.
- •
- Press and hold down the dose button until the dose counter shows “0”. See figure N. The “0” must line up with the dose pointer. You may then hear or feel a “click”.
- •
- Continue to hold the needle in your skin.
- If “0” does not appear in the dose counter after continuously pressing the dose button, your needle may be blocked or damaged, see Frequently Asked Questions.
- •
- Keep the needle in your skin after the dose counter has returned to “0”. Count slowly to 6 to ensure that the full dose has been delivered. See figure O.
- •
- Carefully remove the needle from your skin. See figure P. If blood appears at the injection site, press lightly with a gauze pad. Do not rub the area.
 You may see a drop of Norditropin at the needle tip after injecting. This is normal and does not affect your dose.
You may see a drop of Norditropin at the needle tip after injecting. This is normal and does not affect your dose.
Step 5. After your injection
- •
- Carefully remove the needle from the Pen by turning the needle counterclockwise. See figure Q.
- •
- Place the needle in a sharps disposal container immediately to reduce the risk of a needle stick. See figure R.
 Always dispose of the needle after each injection.
Always dispose of the needle after each injection.
- For further information about safe sharps disposal, see Frequently Asked Questions.
 Do not try to put the needle cap back on . You may stick yourself with the needle.
Do not try to put the needle cap back on . You may stick yourself with the needle.
- •
- Put the Pen cap on your Pen after each use to protect the Norditropin from direct light. See figure S.
- See “How should I store Norditropin?”.
- Always remove the needle from your Pen. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
- How should I store Norditropin?
- •
- Before you use Norditropin FlexPro pens for the first time:
- o
- Store your new, unused Norditropin pen in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- o
- Do not freeze Norditropin.
- o
- Keep Norditropin away from direct light.
- o
- Do not use Norditropin that has been frozen or in temperatures warmer than 77ºF (25ºC).
- o
- Do not use Norditropin after the expiration date printed on the carton and the pen.
- •
- After you use Norditropin FlexPro pens and there is still medicine left:
- o
- Store remaining Norditropin in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC) and use within 4 weeks, or
- o
- Store remaining Norditropin at room temperature no warmer than 77ºF (25ºC) and use within 3 weeks.
Keep Norditropin and all medicines out of the reach of children.
Frequently Asked Questions
How do I see how much Norditropin is left in my Pen?
The Pen scale shows you approximately how much Norditropin is left in your Pen. See figure T below.
To see how much Norditropin is left in your Pen, use the dose counter: Turn the dose selector clockwise until the dose counter stops. The dose pointer will line up with the number of “mg” left in the Pen. You can select a maximum dose of 4.0 mg. If the dose counter stops with the dose pointer lined up with “4.0” , at least 4.0 mg are left in your Pen.
If the dose counter stops with the dose pointer lined up with “2.4”, only 2.4 mg are left in your Pen. See figure U below.
What if I need a larger dose than what is left in my Pen?
It is not possible to select a larger dose on the dose counter than the number of “mg” left in your Pen.
If you need more Norditropin than you have left in your Pen, you can use a new Pen or split your dose between your current Pen and a new Pen. Only split your dose if you have been trained or advised by your healthcare provider on how to do this. You may find it helpful to use a calculator to plan the doses as instructed by your healthcare provider.
Be very careful to calculate your split dose correctly so that you do not give the wrong dose. If you are not sure how to split your dose using two Pens, then select and inject the dose you need with a new Pen.
What if no Norditropin appears when I check the flow?
A. Your needle may be blocked or damaged, if no Norditropin appears at the needle tip. Remove the needle as described in step 5 and repeat steps 1 and 2.
B. Your Pen may be defective, if Norditropin still does not appear after changing the needle. Do not use the Pen. Contact Novo Nordisk at 1-888-668-6444.
What if “0” does not appear after completing my injection?
The needle may be blocked or damaged, and you have not received any Norditropin – even though the dose counter has moved from the dose that you have set. Remove the needle as described in step 5 and repeat steps 1 to 4.
If “0” still does not appear after completing the injection, contact Novo Nordisk at 1-888-668-6444.
How should I take care of my Pen?
Be careful not to drop your Pen or knock it against hard surfaces. Do not expose your Pen to dust, dirt, liquid, or direct light.
See “How should I store Norditropin?”.
Do not try to refill your Pen, it is already prefilled. When your Pen is empty, throw it away and use a new pen. See “How do I dispose of used needles and Pens?”.
Frequently Asked Questions
What if I drop my Pen?
If you drop your Pen or think that something is wrong with it, attach a new disposable needle and check the Norditropin flow before you inject, see steps 1 and 2. Do not try to repair your Pen or pull it apart.
How do I clean my Pen?
Do not wash, soak, or lubricate your Pen. If necessary, clean it with mild detergent on a moistened cloth.
How do I dispose of used needles and Pens?
Put your used needles in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- •
- made of a heavy-duty plastic,
- •
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- •
- upright and stable during use,
- •
- leak-resistant, and
- •
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should dispose of used needles and Pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
When there is not enough medicine left in your Pen for your prescribed dose, the Pen may be thrown away in your household trash after you have removed the needle.
- •
- Caregivers must be very careful when handling needles to reduce the risk of needle sticks and infection.
- •
- Norditropin® FlexPro® 10 mg/1.5 mL Pen is compatible with FlexPro® PenMate®.
www.norditropin.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
PATENT Information: http://novonordisk-us.com/patients/products/product-patents.html
Norditropin® and FlexPro® are registered trademarks of Novo Nordisk Health Care AG.
Novo Nordisk® and PenMate®are registered trademarks of Novo Nordisk A/S.
© 2004-2020 Novo Nordisk Health Care AG
For further information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-888-668-6444
norditropin-us.com
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark
Revised 3/2020
INSTRUCTIONS FOR USE
Norditropin® (Nor-dee-tro-pin) FlexPro®
(somatropin) injection
15 mg/1.5 mL
Norditropin FlexPro Pen is for single-patient-use only.
Supplies you will need:
- •
- Norditropin FlexPro prefilled Pen new injection needle. Norditropin prefilled Pen is designed to be used with all Novo Nordisk disposable needles up to a length of 8 mm
- •
- sharps disposal container. See step 5 for information on how to throw away (dispose of) used needles and Pens.
- •
- alcohol pad
- •
- gauze pad
How to use your Norditropin FlexPro Pen
5 steps you should follow for a Norditropin injection:
- Step 1: Prepare your Norditropin FlexPro Pen
- Step 2: Check the Norditropin flow with each new Pen
- Step 3: Select your dose
- Step 4: Inject your dose
- Step 5: After your injection
For further information about your Pen see:
- Frequently Asked Questions
- Important information
- Patient Information
Make sure that you read this information carefully.
Norditropin is for use under the skin only (subcutaneous).
Do not share your Norditropin Pen and needles with another person. You may give another person an infection or get an infection from them.
Do not use your Pen without proper training from your healthcare provider.
Make sure that you are confident in giving an injection with the Pen before you start your treatment.
If you are blind or have poor eyesight and cannot read the dose counter on the Pen, do not use this Pen without help. Get help from a person with good eyesight who is trained to use the Pen.
Step 1. Prepare your Norditropin FlexPro Pen
- •
- Wash your hands with soap and water.
- •
- Check the name, strength, and colored label on your Pen to make sure that it contains Norditropin in the right strength.
- •
- Pull off the Pen cap.
- •
- Turn the Pen upside down 1 or 2 times to check that the Norditropin in your Pen is clear and colorless.
- See figure A. If the Norditropin looks cloudy, do not use the Pen.
- •
- When you are ready to give your injection, take a new disposable needle, and remove the paper tab.
- •
- Push the needle straight onto the Pen. Turn the needle clockwise until it is on tight. See figure B.
- Always use a new needle for each injection. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
- •
- Pull off the outer needle cap and dispose of it. See figure C.
- •
- Pull off the inner needle cap and dispose of it. See figure D.
- A drop of Norditropin may appear at the needle tip. This is normal, but you must still check the Norditropin flow with each new Pen. See step 2.
- Never use a bent or damaged needle.
Step 2. Check the Norditropin flow with each new Pen
 If your Pen is already in use, go to step 3.
If your Pen is already in use, go to step 3.
- Before using a new Pen, check the Norditropin flow to make sure the growth hormone can flow through the Pen and needle.
- •
- Turn the dose selector clockwise 1 tick marking on the dose counter to select 0.1 mg. You will hear a faint “click” when you turn the dose selector. See figure E.
- •
- 1 marking on the dose counter equals 0.1 mg. See figure F.
- •
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter returns to “0”. The “0” must line up with the dose pointer. See figure G.
- •
- Check that a drop of Norditropin appears at the needle tip. See figure H.
 If no Norditropin appears, repeat step 2 up to 6 times.
If no Norditropin appears, repeat step 2 up to 6 times.
If you still do not see a drop of Norditropin, change the needle:
- •
- Carefully remove the needle from the Pen by turning the needle counterclockwise. Place the needle in a sharps disposal container immediately. See step 5.
- •
- and repeat step 2 again.
- Do not use the Pen if a drop of Norditropin still does not appear after changing the needle and repeating step 2. Call Novo Nordisk at 1-888-668-6444 for help.
Step 3. Select your dose
- •
- To start, check that the dose pointer is set at “0”.
- •
- Turn the dose selector clockwise to select the dose you need. See figure I.
When you have selected your dose, you can go to step 4.
 If there is not enough Norditropin left to select a full dose, see Frequently Asked Questions.
If there is not enough Norditropin left to select a full dose, see Frequently Asked Questions.
- The dose counter shows the dose in “mg”. See figures J and K. Always use the dose counter to select the exact dose. Do not use the “click” sounds you hear when you turn the dose selector or the Pen scale to select your dose. Only the dose pointer on the dose counter will show the exact dose selected.
- If you select the wrong dose, you can turn the dose selector clockwise or counterclockwise to the correct dose. See figure L.
- The Pen “clicks” sound and feel differently when the dose selector is turned clockwise, counterclockwise, or if you forcefully move it past the number of “mg” left in the Pen.
Step 4. Inject your dose
- •
- Select the injection site.
- •
- Norditropin can be injected under the skin (subcutaneously) of your hips, stomach area (abdomen), buttocks, upper legs (thighs), or upper arms, as instructed by your healthcare provider. Change the injection site every day.
- •
- Wipe the injection site with an alcohol swab and let the area dry.
- •
- Insert the needle into your skin as your healthcare provider has shown you. See figure M.
- Make sure you can see the dose counter. Do not cover it with your fingers. This could block the injection.
- •
- Press and hold down the dose button until the dose counter shows “0”. See figure N. The “0” must line up with the dose pointer. You may then hear or feel a “click”.
- •
- Continue to hold the needle in your skin.
- If “0” does not appear in the dose counter after continuously pressing the dose button, your needle may be blocked or damaged, see Frequently Asked Questions.
- •
- Keep the needle in your skin after the dose counter has returned to “0”. Count slowly to 6 to ensure that the full dose has been delivered. See figure O.
- •
- Carefully remove the needle from your skin. See figure P. If blood appears at the injection site, press lightly with a gauze pad. Do not rub the area.
 You may see a drop of Norditropin at the needle tip after injecting. This is normal and does not affect your dose.
You may see a drop of Norditropin at the needle tip after injecting. This is normal and does not affect your dose.
Step 5. After your injection
- •
- Carefully remove the needle from the Pen by turning the needle counterclockwise. See figure Q.
- •
- Place the needle in a sharps disposal container immediately to reduce the risk of a needle stick. See figure R.
 Always dispose of the needle after each injection.
Always dispose of the needle after each injection.
- For further information about safe sharps disposal, see Frequently Asked Questions.
 Do not try to put the needle cap back on . You may stick yourself with the needle.
Do not try to put the needle cap back on . You may stick yourself with the needle.
- •
- Put the Pen cap on your Pen after each use to protect the Norditropin from direct light. See figure S.
- See “How should I store Norditropin?”.
- Always remove the needle from your Pen. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
- How should I store Norditropin?
- •
- Before you use Norditropin FlexPro pens for the first time:
- o
- Store your new, unused Norditropin pen in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- o
- Do not freeze Norditropin.
- o
- Keep Norditropin away from direct light.
- o
- Do not use Norditropin that has been frozen or in temperatures warmer than 77ºF (25ºC).
- o
- Do not use Norditropin after the expiration date printed on the carton and the pen.
- •
- After you use Norditropin FlexPro pens and there is still medicine left:
- o
- Store remaining Norditropin in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC) and use within 4 weeks, or
- o
- Store remaining Norditropin at room temperature no warmer than 77ºF (25ºC) and use within 3 weeks.
Keep Norditropin and all medicines out of the reach of children.
Frequently Asked Questions
How do I see how much Norditropin is left in my Pen?
The Pen scale shows you approximately how much Norditropin is left in your Pen. See figure T below.
To see how much Norditropin is left in your Pen, use the dose counter: Turn the dose selector clockwise until the dose counter stops. The dose pointer will line up with the number of “mg” left in the Pen. You can select a maximum dose of 8.0 mg. If the dose counter stops with the dose pointer lined up with “8.0” , at least 8.0 mg are left in your Pen.
If the dose counter stops with the dose pointer lined up with “3.8”, only 3.8 mg are left in your Pen. See figure U below.
What if I need a larger dose than what is left in my Pen?
It is not possible to select a larger dose on the dose counter than the number of “mg” left in your Pen.
If you need more Norditropin than you have left in your Pen, you can use a new Pen or split your dose between your current Pen and a new Pen. Only split your dose if you have been trained or advised by your healthcare provider on how to do this. You may find it helpful to use a calculator to plan the doses as instructed by your healthcare provider.
Be very careful to calculate your split dose correctly so that you do not give the wrong dose. If you are not sure how to split your dose using two Pens, then select and inject the dose you need with a new Pen.
What if no Norditropin appears when I check the flow?
A. Your needle may be blocked or damaged, if no Norditropin appears at the needle tip. Remove the needle as described in step 5 and repeat steps 1 and 2.
B. Your Pen may be defective, if Norditropin still does not appear after changing the needle. Do not use the Pen. Contact Novo Nordisk at 1-888-668-6444.
What if “0” does not appear after completing my injection?
The needle may be blocked or damaged, and you have not received any Norditropin – even though the dose counter has moved from the dose that you have set. Remove the needle as described in step 5 and repeat steps 1 to 4.
If “0” still does not appear after completing the injection, contact Novo Nordisk at 1-888-668-6444.
How should I take care of my Pen?
Be careful not to drop your Pen or knock it against hard surfaces. Do not expose your Pen to dust, dirt, liquid, or direct light.
See “How should I store Norditropin?”.
Do not try to refill your Pen, it is already prefilled. When your Pen is empty, throw it away and use a new pen. See “How do I dispose of used needles and Pens?”.
Frequently Asked Questions
What if I drop my Pen?
If you drop your Pen or think that something is wrong with it, attach a new disposable needle and check the Norditropin flow before you inject, see steps 1 and 2. Do not try to repair your Pen or pull it apart.
How do I clean my Pen?
Do not wash, soak, or lubricate your Pen. If necessary, clean it with mild detergent on a moistened cloth.
How do I dispose of used needles and Pens?
Put your used needles in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- •
- made of a heavy-duty plastic,
- •
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- •
- upright and stable during use,
- •
- leak-resistant, and
- •
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should dispose of used needles and Pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
When there is not enough medicine left in your Pen for your prescribed dose, the Pen may be thrown away in your household trash after you have removed the needle.
- •
- Caregivers must be very careful when handling needles to reduce the risk of needle sticks and infection.
- •
- Norditropin® FlexPro® 15 mg/1.5 mL Pen is compatible with FlexPro® PenMate®.
www.norditropin.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
PATENT Information: http://novonordisk-us.com/patients/products/product-patents.html
Norditropin® and FlexPro® are registered trademarks of Novo Nordisk Health Care AG.
Novo Nordisk® and PenMate®are registered trademarks of Novo Nordisk A/S.
© 2004-2020 Novo Nordisk Health Care AG
For further information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-888-668-6444
norditropin-us.com
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark
Revised 3/2020
INSTRUCTIONS FOR USE
Norditropin® (Nor-dee-tro-pin) FlexPro®
(somatropin) injection
30 mg/3 mL
Norditropin FlexPro Pen is for single-patient-use only.
Supplies you will need:
- •
- Norditropin FlexPro prefilled Pen new injection needle. Norditropin prefilled Pen is designed to be used with all Novo Nordisk disposable needles up to a length of 8 mm
- •
- sharps disposal container. See step 5 for information on how to throw away (dispose of) used needles and Pens.
- •
- alcohol pad
- •
- gauze pad
How to use your Norditropin FlexPro Pen
5 steps you should follow for a Norditropin injection:
- Step 1: Prepare your Norditropin FlexPro Pen
- Step 2: Check the Norditropin flow with each new Pen
- Step 3: Select your dose
- Step 4: Inject your dose
- Step 5: After your injection
For further information about your Pen see:
- Frequently Asked Questions
- Important information
- Patient Information
Make sure that you read this information carefully.
Norditropin is for use under the skin only (subcutaneous).
Do not share your Norditropin Pen and needles with another person. You may give another person an infection or get an infection from them.
Do not use your Pen without proper training from your healthcare provider.
Make sure that you are confident in giving an injection with the Pen before you start your treatment.
If you are blind or have poor eyesight and cannot read the dose counter on the Pen, do not use this Pen without help. Get help from a person with good eyesight who is trained to use the Pen.
Step 1. Prepare your Norditropin FlexPro Pen
- •
- Wash your hands with soap and water.
- •
- Check the name, strength, and colored label on your Pen to make sure that it contains Norditropin in the right strength.
- •
- Pull off the Pen cap.
- •
- Turn the Pen upside down 1 or 2 times to check that the Norditropin in your Pen is clear and colorless.
- See figure A. If the Norditropin looks cloudy, do not use the Pen.
- •
- When you are ready to give your injection, take a new disposable needle, and remove the paper tab.
- •
- Push the needle straight onto the Pen. Turn the needle clockwise until it is on tight. See figure B.
 Always use a new needle for each injection. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
Always use a new needle for each injection. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
- •
- Pull off the outer needle cap and dispose of it. See figure C.
- •
- Pull off the inner needle cap and dispose of it. See figure D.
- A drop of Norditropin may appear at the needle tip. This is normal, but you must still check the Norditropin flow with each new Pen. See step 2.
- Never use a bent or damaged needle.
Step 2. Check the Norditropin flow with each new Pen
 If your Pen is already in use, go to step 3.
If your Pen is already in use, go to step 3.
- Before using a new Pen, check the Norditropin flow to make sure the growth hormone can flow through the Pen and needle.
- •
- Turn the dose selector clockwise 1 tick marking on the dose counter to select 0.1 mg. You will hear a faint “click” when you turn the dose selector. See figure E.
- •
- 1 marking on the dose counter equals 0.1 mg. See figure F.
- •
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter returns to “0”.
- The “0” must line up with the dose pointer. See figure G.
- •
- Check that a drop of Norditropin appears at the needle tip. See figure H.
 If no Norditropin appears, repeat step 2 up to 6 times.
If no Norditropin appears, repeat step 2 up to 6 times.
If you still do not see a drop of Norditropin, change the needle:
- •
- Carefully remove the needle from the Pen by turning the needle counterclockwise. Place the needle in a sharps disposal container immediately. See step 5.
- •
- and repeat step 2 again.
- Do not use the Pen if a drop of Norditropin still does not appear after changing the needle and repeating step 2. Call Novo Nordisk at 1-888-668-6444 for help.
Step 3. Select your dose
- •
- To start, check that the dose pointer is set at “0”.
- •
- Turn the dose selector clockwise to select the dose you need. See figure I.
When you have selected your dose, you can go to step 4.
 If there is not enough Norditropin left to select a full dose, see Frequently Asked Questions.
If there is not enough Norditropin left to select a full dose, see Frequently Asked Questions.
- The dose counter shows the dose in “mg”. See figures J and K. Always use the dose counter to select the exact dose. Do not use the “click” sounds you hear when you turn the dose selector or the Pen scale to select your dose. Only the dose pointer on the dose counter will show the exact dose selected.
- If you select the wrong dose, you can turn the dose selector clockwise or counterclockwise to the correct dose. See figure L.
- The Pen “clicks” sound and feel differently when the dose selector is turned clockwise, counterclockwise, or if you forcefully move it past the number of “mg” left in the Pen.
Step 4. Inject your dose
- •
- Select the injection site.
- •
- Norditropin can be injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs (thighs), or upper arms, as instructed by your healthcare provider. Change the injection site every day.
- •
- Wipe the injection site with an alcohol swab and let the area dry.
- •
- Insert the needle into your skin as your healthcare provider has shown you. See figure M.
- Make sure you can see the dose counter. Do not cover it with your fingers. This could block the injection.
- •
- Press and hold down the dose button until the dose counter shows “0”. See figure N. The “0” must line up with the dose pointer. You may then hear or feel a “click”.
- •
- Continue to hold the needle in your skin.
- If “0” does not appear in the dose counter after continuously pressing the dose button, your needle may be blocked or damaged, see Frequently Asked Questions.
- •
- Keep the needle in your skin after the dose counter has returned to “0”. Count slowly to 6 to ensure that the full dose has been delivered. See figure O.
- •
- Carefully remove the needle from your skin. See figure P. If blood appears at the injection site, press lightly with a gauze pad. Do not rub the area.
 You may see a drop of Norditropin at the needle tip after injecting. This is normal and does not affect your dose.
You may see a drop of Norditropin at the needle tip after injecting. This is normal and does not affect your dose.
Step 5. After your injection
- •
- Carefully remove the needle from the Pen by turning the needle counterclockwise. See figure Q.
- •
- Place the needle in a sharps disposal container immediately to reduce the risk of a needle stick. See figure R.
 Always dispose of the needle after each injection.
Always dispose of the needle after each injection.
- For further information about safe sharps disposal, see Frequently Asked Questions.
 Do not try to put the needle cap back on . You may stick yourself with the needle.
Do not try to put the needle cap back on . You may stick yourself with the needle.
- •
- Put the Pen cap on your Pen after each use to protect the Norditropin from direct light. See figure S.
- See “How should I store Norditropin?”.
 Always remove the needle from your Pen. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
Always remove the needle from your Pen. This reduces the risk of contamination, infection, leakage of Norditropin, and blocked needles leading to incorrect dosing.
- How should I store Norditropin?
- •
- Before you use Norditropin FlexPro pens for the first time:
- o
- Store your new, unused Norditropin pen in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- o
- Do not freeze Norditropin.
- o
- Keep Norditropin away from direct light.
- o
- Do not use Norditropin that has been frozen or in temperatures warmer than 77ºF (25ºC).
- o
- Do not use Norditropin after the expiration date printed on the carton and the pen.
- •
- After you use Norditropin FlexPro pens and there is still medicine left:
- o
- Store remaining Norditropin in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC) and use within 4 weeks, or
- o
- Store remaining Norditropin at room temperature no warmer than 77ºF (25ºC) and use within 3 weeks.
Keep Norditropin and all medicines out of the reach of children.
Frequently Asked Questions
How do I see how much Norditropin is left in my Pen?
The Pen scale shows you approximately how much Norditropin is left in your Pen. See figure T below.
To see how much Norditropin is left in your Pen, use the dose counter: Turn the dose selector clockwise until the dose counter stops. The dose pointer will line up with the number of “mg” left in the Pen. You can select a maximum dose of 8.0 mg. If the dose counter stops with the dose pointer lined up with “8.0” , at least 8.0 mg are left in your Pen.
If the dose counter stops with the dose pointer lined up with “3.8”, only 3.8 mg are left in your Pen. See figure U below.
What if I need a larger dose than what is left in my Pen?
It is not possible to select a larger dose on the dose counter than the number of “mg” left in your Pen.
If you need more Norditropin than you have left in your Pen, you can use a new Pen or split your dose between your current Pen and a new Pen. Only split your dose if you have been trained or advised by your healthcare provider on how to do this. You may find it helpful to use a calculator to plan the doses as instructed by your healthcare provider.
Be very careful to calculate your split dose correctly so that you do not give the wrong dose. If you are not sure how to split your dose using two Pens, then select and inject the dose you need with a new Pen.
What if no Norditropin appears when I check the flow?
A. Your needle may be blocked or damaged, if no Norditropin appears at the needle tip. Remove the needle as described in step 5 and repeat steps 1 and 2.
B. Your Pen may be defective, if Norditropin still does not appear after changing the needle. Do not use the Pen. Contact Novo Nordisk at 1-888-668-6444.
What if “0” does not appear after completing my injection?
The needle may be blocked or damaged, and you have not received any Norditropin – even though the dose counter has moved from the dose that you have set. Remove the needle as described in step 5 and repeat steps 1 to 4.
If “0” still does not appear after completing the injection, contact Novo Nordisk at 1-888-668-6444.
How should I take care of my Pen?
Be careful not to drop your Pen or knock it against hard surfaces. Do not expose your Pen to dust, dirt, liquid, or direct light.
See “How should I store Norditropin?”.
Do not try to refill your Pen, it is already prefilled. When your Pen is empty, throw it away and use a new pen. See “How do I dispose of used needles and Pens?”.
Frequently Asked Questions
What if I drop my Pen?
If you drop your Pen or think that something is wrong with it, attach a new disposable needle and check the Norditropin flow before you inject, see steps 1 and 2. Do not try to repair your Pen or pull it apart.
How do I clean my Pen?
Do not wash, soak, or lubricate your Pen. If necessary, clean it with mild detergent on a moistened cloth.
How do I dispose of used needles and Pens?
Put your used needles in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
•made of a heavy-duty plastic,
•can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
•upright and stable during use,
•leak-resistant, and
•properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should dispose of used needles and Pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
When there is not enough medicine left in your Pen for your prescribed dose, the Pen may be thrown away in your household trash after you have removed the needle.
- •
- Caregivers must be very careful when handling needles to reduce the risk of needle sticks and infection.
- •
- Norditropin® FlexPro® 30 mg/3 mL Pen is not compatible with FlexPro® PenMate®.
www.norditropin.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
PATENT Information: http://novonordisk-us.com/patients/products/product-patents.html
Norditropin® and FlexPro® are registered trademarks of Novo Nordisk Health Care AG.
Novo Nordisk® and PenMate®are registered trademarks of Novo Nordisk A/S.
© 2004-2020 Novo Nordisk Health Care AG
For further information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-888-668-6444
norditropin-us.com
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark
Revised 3/2020
INSTRUCTIONS FOR USE
Norditropin® (Nor-dee-tro-pin) FlexPro®
(somatropin) injection
Prefilled Pen with PenMate®
Read this Instructions for Use before you start using your Pen with PenMate.
- • PenMate hides the needle when you inject your Norditropin growth hormone with Norditropin FlexPro 5 mg, 10 mg, and 15 mg Pens so that you cannot see it. Use your PenMate only after you have been trained by a healthcare provider.
- • Blind people or people with severe vision problems should only use the PenMate and Pen with help from another person with good eyesight who is trained to use the PenMate and Pen.
- • The figures in these instructions show PenMate being used with a Norditropin FlexPro 5 mg Pen and a NovoFine needle that is 8 mm long. Even if you are using a 10 mg or 15 mg Pen or a different needle that is 8 mm long the instructions are the same.
- • Do not share your Norditropin Pen and needles with another person. You may give another person an infection or get an infection from them.
Supplies you will need to use your Pen with PenMate:
- • 1 PenMate. See figure A .
- • 1 Norditropin FlexPro Pen. See figure B . PenMate does not work with other injection devices.
- • 1 disposable needle up to a length of 8 mm. See figure C . Needles are not included with your PenMate or Pen.
- • 2 alcohol swabs. See figure C .
- • a sharps disposal container. See figure C . See ”How should I dispose of my Pen and needles” at the end of these instructions for information on how to dispose of used needles.
Figure A
Figure B
Figure C
Figure D
Step 1: Preparing your Pen with PenMate:
Wash your hands with soap and water and dry them. Check the name and the colored label on your Pen to make sure it contains the growth hormone strength prescribed by your healthcare provider.
Pull off the PenMate cap.
See figure E.
Figure E
- Pull off the Pen cap and throw it away.
- See figure F.
You will not need the Pen cap with your PenMate.
Figure F
Look in the Pen window. Check that the liquid medicine in your Pen is clear and colorless by tipping it upside down 1 or 2 times.
See figure G.
If the liquid looks cloudy or unclear, do not use the Pen.
Figure G
- Wipe the front stopper on the needle thread of the Pen with an alcohol swab.
- See figure H.
Figure H
- Insert the Pen into the PenMate. Twist the Pen clockwise until you hear or feel a click.
- See figure I.
The Pen is correctly attached in your PenMate when the display window on the Pen lines up with the insertion button on your PenMate.
Figure I
Step 2. Attaching the needle to your Pen:
- •
- Do not place a needle on your Pen until you are ready to give an injection.
- •
- Always use a new needle for each injection.
- •
- Do not use a bent or damaged needle.
Take a new disposable needle and tear off the paper tab.
See figure J.
Figure J
Holding the Pen with 1 hand, firmly press the needle onto the needle thread of the Pen. Screw the needle in a clockwise direction until the needle will not turn anymore.
See figure K.
Figure K
Pull off the outer needle cap and save it.
See figure L.
You will need the outer needle cap after the injection so you can safely remove the needle from the Pen.
Figure L
- Pull off the inner needle cap and throw it away.
- See figure M.
A drop of liquid may appear at the needle tip. This is normal.
Figure M
Step 3. Priming a new Pen:
Checking the growth hormone flow in the Pen (priming) is not needed for a Pen you have used before. If the Pen has already been primed, go to Step 4.
Before you use a new Pen you must prepare it for use. Hold the Pen with 1 hand and turn the dose selector clockwise 1 tick mark to select the minimum dose.
See figure N.
You may hear or feel a click when you turn the dose selector.
Figure N
When you turn the dose selector 1 tick mark, you select the smallest amount of medicine for a dose.
See figure O.
Figure O
This lowest dose will be used for your Norditropin flow check dose.
Hold your Pen with PenMate with the needle pointing up. You may see air bubbles in the PenMate window. Gently tap the top of PenMate a few times to let any air bubbles rise to the top.
See figure P.
Figure P
Press the dose button until the dose pointer lines up with the “0” in the display window on the Pen and a drop of liquid appears at the needle tip.
See figure Q.
Figure Q
If no drop of liquid appears at the needle tip, repeat Step 3 again up to 6 times.
If there is still no drop of liquid at the needle tip, change the needle and repeat Step 3 again.
If a drop of liquid still does not appear at the needle tip after repeating Step 3 and changing the needle, call Novo Nordisk at 1-888-668-6444 for assistance.
Step 4. Selecting the correct dose of Norditropin:
Use the dose selector on your Pen to make sure you have the exact dose selected. Your dose will be in a certain number of mg (milligrams).
To start, check that the dose pointer on the Pen is set at “0”.
Select the dose you need by turning the dose selector clockwise. If you go beyond your dose, turn the dose selector counterclockwise until the right number of mg lines up with the dose pointer. See figure R.
Figure R
To guide you, the dose selector click sound is different when turned clockwise (softer click) or counterclockwise (louder click). You will hear a click for every single unit dialed.
When dialing counterclockwise, be careful not to press the dose button as liquid will come out.
You can use the growth hormone scale on the side of the Pen to see approximately how much growth hormone is left in the Pen. You can also use the dose selector to see exactly how much growth hormone is left in the Pen.
If the Pen contains less than 2 mg, 4 mg, or 8 mg (depending on whether you use a 5 mg, 10 mg, or 15 mg Pen), turn the dose selector until it stops. The number that lines up with the dose pointer shows how many mg are left in the Pen. You cannot set a dose higher than the number of mg left in the Pen.
If there is not enough Norditropin left in the Pen for your full dose, use a new Norditropin FlexPro Pen to inject the remaining amount of your dose or contact your healthcare provider.
Remember to subtract the dose already received. For example, if the dose is 0.7 mg and you can only set the dose selector to 0.35 mg, you should inject another 0.35 mg with a new Norditropin FlexPro Pen.
Important:
Do not use the Pen clicks to count the number of mg you select. Only the display window and dose pointer will show the exact number.
Do not use the growth hormone scale to measure how much liquid to inject. Only the display window and dose pointer will show the exact number.
Step 5. Selecting your injection site and injecting the dose of Norditropin:
Change your injection site every day. Select the injection site and wipe your skin with an alcohol swab as your healthcare provider showed you.
Norditropin can be injected under your skin (subcutaneously) of your hips, stomach area
(abdomen), upper legs (thighs), upper arms, or as otherwise instructed by your healthcare provider.
See Figure S.
Figure S
Hold onto both the PenMate and your Pen without touching the insertion button on the PenMate or the dose button on the Pen.
Do not press the insertion button on the PenMate before you are ready to inject your dose. This lowers the risk of hurting yourself with the needle.
Hold the PenMate firmly with 1 hand and pull the Pen out with your other hand until you hear and feel a click. See figure T.
The needle is now hidden in PenMate.
Figure T
Norditropin is for use under your skin only (subcutaneous). Hold the PenMate against your skin. Press the insertion button on the PenMate until you hear or feel a click.
When you hear or feel the click, the needle has been inserted automatically into your skin.
See figure U.
You are now ready to inject your dose.
Figure U
Press the dose button on the Pen to inject your dose. Do not turn the dose button while you are pressing it. If you turn the dose button, you will not inject growth hormone.
Make sure you can see the display window. Do not cover it with your fingers.
Press and hold down the dose button on the Pen until the display window returns to “0”.
The “0” must line up with the dose pointer. You may then hear or feel a firm click.
See figure V.
Figure V
If the dose button cannot be pushed in completely or “0” does not appear in the display window, you did not receive the full dose. Call Novo Nordisk at
1-888-668-6444 for assistance. You may need a new Pen.
After the display window has returned to “0”, leave the needle under your skin for at least 6 seconds to make sure you get your full dose.
See figure V.
Let go of the dose button while you wait.
Important:
Always press the dose button to inject the dose. Turning the dose selector will not inject the dose.
Do not touch the display window when you inject, as this can block the injection.
Carefully lift the Pen to remove the needle from the skin.
See figure W.
Figure W
- Step 6. What to do after your injection is completed:
Carefully put the outer needle cap back on the needle.
Remove the needle from the Pen after each injection.
See figure X.
Figure X
Unscrew the needle by turning it counterclockwise. Do not touch the needle. Hold the Pen with 1 hand and carefully remove the needle from the Pen with your other hand.
See figure Y.
Dispose of the needle as directed by a healthcare provider. See “How should I dispose of my Pen and needles?” at the end of these instructions.
Figure Y
Put the PenMate cap back on your PenMate after each use to protect the growth hormone from light.
See figure Z.
Figure Z
Important safety information to remember:
- •
- Be careful not to drop your PenMate and Pen or knock them against a hard surface. If this happens you will need to check the growth hormone flow.
- •
- Do not try to put the inner needle cap back on the needle. You may stick yourself with the needle. Be careful when handling used needles to avoid needle stick injuries.
- •
- After each use always remove and dispose of the needle from your Pen.
- •
- Do not share your Pen or needles with other people.
- •
- If your PenMate is damaged or lost, you can still use your Pen without your PenMate.
- •
- Always keep your Pen and needles out of reach of others, especially children.
How should I replace an empty Pen?
PenMate is reusable and should not be disposed of. Reuse your PenMate by replacing your Pen when it is empty.
When your Pen is empty, twist the Pen until you hear or feel a click.
See figure AA.
Figure AA
Gently pull the Pen out of PenMate.
See figure BB.
Before disposing of your empty Pen, make sure the needle has been removed. Dispose of the empty Pen as recommended by your healthcare provider. See “How should I dispose of my Pen and needles?” at the end of these instructions.
Figure BB
Insert the new Pen into your PenMate.
See figure CC.
Figure CC
Twist the Pen until you hear or feel a click.
See figure DD.
The Pen is correctly attached in your PenMate when the display window on the Pen lines up with the insertion button on your PenMate.
Figure DD
How should I store my PenMate and Pen?
- •
- Do not expose your PenMate or Pen to dust, dirt, or any kind of liquid.
- •
- Store your PenMate and Pen in their case. See figure D at the beginning of these instructions.
- •
- When your Pen is inserted in PenMate, store it as described in the Patient Information Leaflet that comes with your Pen.
How should I care for and clean my Pen with PenMate?
- •
- Do not try to refill your Pen. It is prefilled.
- •
- Do not try to repair your PenMate or your Pen.
- •
- Only clean your PenMate or Pen with a mild detergent on a moistened cloth.
- •
- Do not wash, soak, or lubricate your PenMate or Pen. Do not use products containing bleaching agents, such as chlorine, iodine, or alcohol to clean your PenMate or Pen. These products may damage them.
- •
- If there is liquid growth hormone on the outside of your PenMate or Pen, clean it with a mild detergent on a moistened cloth before it dries up.
How should I dispose of my Pen and needles?
- •
- Put your used needles and Pens in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and Pens in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and Pens. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Need help?
PenMate must only be used according to the instructions provided. The manufacturer cannot be held responsible for any problems with PenMate if these instructions have not been followed.
If you find that your PenMate or case is defective, make sure to have Novo Nordisk replace it. Call the number below to order a new PenMate or case and arrange return of the defective item for inspection.
For assistance or further information, write to:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
Visit norditropin-us.com
Or call: 1-888-668-6444
PATENT Information: http://novonordisk-us.com/patients/products/product-patents.html
Novo Nordisk®, PenMate® and NovoFine® are registered trademarks of Novo Nordisk A/S.
Norditropin® and FlexPro® are registered trademarks of Novo Nordisk Health Care AG.
© 2015-2017 Novo Nordisk A/S
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
Revised: 08/2017
PRINCIPAL DISPLAY PANEL - NORDITROPIN FLEXPRO 5 MG/1.5 ML
PRINCIPAL DISPLAY PANEL - NORDITROPIN FLEXPRO 10 MG/1.5 ML
PRINCIPAL DISPLAY PANEL - NORDITROPIN FLEXPRO 15 MG/1.5 ML
PRINCIPAL DISPLAY PANEL - NORDITROPIN FLEXPRO 30 MG/3 ML
INGREDIENTS AND APPEARANCE
| NORDITROPIN
somatropin injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NORDITROPIN
somatropin injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NORDITROPIN
somatropin injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NORDITROPIN
somatropin injection, solution |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Novo Nordisk (622920320) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novo Nordisk A/S - Hax | 306711800 | MANUFACTURE(0169-7704, 0169-7705, 0169-7708, 0169-7703) , API MANUFACTURE(0169-7704, 0169-7705, 0169-7708, 0169-7703) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novo Nordisk A/S - Kv | 311359009 | MANUFACTURE(0169-7704, 0169-7705, 0169-7708, 0169-7703) | |

































 Important information
Important information Additional information
Additional information