Search by Drug Name or NDC
NDC 00363-7007-15 No Drip Anefrin Nasal 0.05 g/100mL Details
No Drip Anefrin Nasal 0.05 g/100mL
No Drip Anefrin Nasal is a NASAL LIQUID in the HUMAN OTC DRUG category. It is labeled and distributed by WALGREENS. The primary component is OXYMETAZOLINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Oxymetazoline nasal spray is used to relieve nasal discomfort caused by colds, allergies, and hay fever. It is also used to relieve sinus congestion and pressure. Oxymetazoline nasal spray should not be used to treat children younger than 6 years of age unless it is recommended by a doctor. Children 6 to 12 years of age should use oxymetazoline nasal spray carefully and under adult supervision. Oxymetazoline is in a class of medications called nasal decongestants. It works by narrowing the blood vessels in the nasal passages.
Related Packages: 00363-7007-15Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Oxymetazoline Nasal Spray
Product Information
| NDC | 00363-7007 |
|---|---|
| Product ID | 0363-7007_bbf0dc69-c2d0-385e-e053-2995a90af9ee |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | No Drip Anefrin Nasal |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | OXYMETAZOLINE HYDROCHLORIDE |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | LIQUID |
| Route | NASAL |
| Active Ingredient Strength | 0.05 |
| Active Ingredient Units | g/100mL |
| Substance Name | OXYMETAZOLINE HYDROCHLORIDE |
| Labeler Name | WALGREENS |
| Pharmaceutical Class | Imidazolines [CS], Increased Sympathetic Activity [PE], Vasoconstriction [PE], Vasoconstrictor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Listing Certified Through | 2022-12-31 |
Package
Package Images
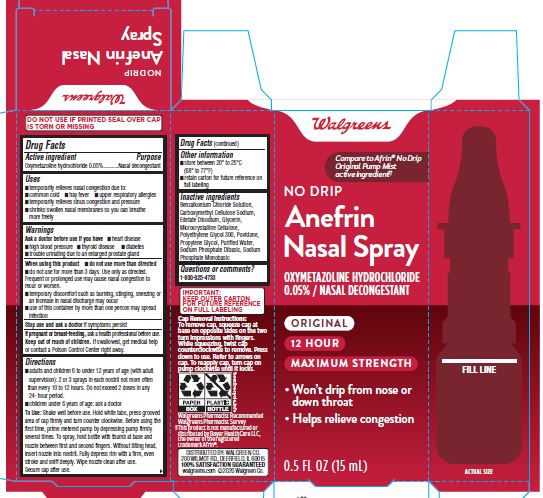
NDC 00363-7007-15 (00363700715)
| NDC Package Code | 0363-7007-15 |
|---|---|
| Billing NDC | 00363700715 |
| Package | 15 mL in 1 BOTTLE (0363-7007-15) |
| Marketing Start Date | 2021-02-26 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b6234e99-115f-6018-e053-2a95a90a6989 Details
Uses
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostrate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as buring, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection
Stop use and ask a doctor if symptoms persist
If pregnant or breast-feeding, ask a health professional before use.
SPL UNCLASSIFIED SECTION
Directions
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- childer under 6 years of age: ask a doctor.
To Use: Shake well before use. Hold white tabs, press grooved area of cap firmly and turn counter clockwise. Before using for the first time, prime metered pump by depressing firmly several times. To spray, hold bottle with thumb at base and nozzle between first and second fingers. Without tilting head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and sniff deeply. Wipe nozel clean after use.
Secure cap after use.
Other information
Inactive ingredients
15 mL Bottle Carton
INGREDIENTS AND APPEARANCE
| NO DRIP ANEFRIN NASAL
oxymetazoline hydrochloride liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - WALGREENS (008965063) |
| Registrant - MEDICAL PRODUCTS LABORATORIES, INC. (002290302) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MEDICAL PRODUCTS LABORATORIES, INC. | 002290302 | manufacture(0363-7007) | |