Search by Drug Name or NDC
NDC 00573-0552-11 PREPARATION H SOOTHING RELIEF ANTI-ITCH 10 mg/g Details
PREPARATION H SOOTHING RELIEF ANTI-ITCH 10 mg/g
PREPARATION H SOOTHING RELIEF ANTI-ITCH is a TOPICAL CREAM in the HUMAN OTC DRUG category. It is labeled and distributed by GlaxoSmithKline Consumer Healthcare Holdings (US) LLC. The primary component is HYDROCORTISONE.
MedlinePlus Drug Summary
Rectal hydrocortisone is used along with other medications to treat proctitis (swelling in the rectum) and ulcerative colitis (a condition which causes swelling and sores in the lining of the large intestine and rectum). It is also used to relieve itching and swelling from hemorrhoids and other rectal problems. Hydrocortisone is in a class of medications called corticosteroids. It works by activating natural substances in the skin to reduce swelling, redness, and itching.
Related Packages: 00573-0552-11Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Hydrocortisone Rectal
Product Information
| NDC | 00573-0552 |
|---|---|
| Product ID | 0573-0552_1e8b9043-b6ab-4bfc-b23b-935e2bd64b52 |
| Associated GPIs | 90550075003720 |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | PREPARATION H SOOTHING RELIEF ANTI-ITCH |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | hydrocortisone |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | CREAM |
| Route | TOPICAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/g |
| Substance Name | HYDROCORTISONE |
| Labeler Name | GlaxoSmithKline Consumer Healthcare Holdings (US) LLC |
| Pharmaceutical Class | Corticosteroid Hormone Receptor Agonists [MoA], Corticosteroid [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part348 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

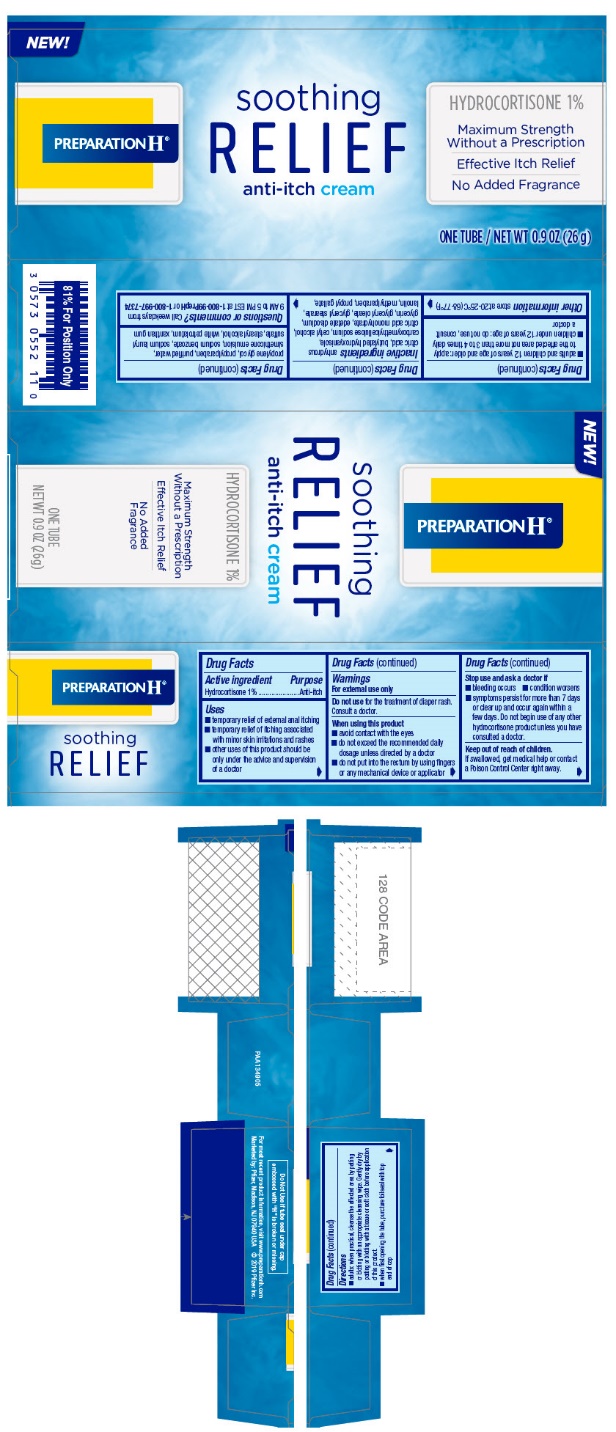
NDC 00573-0552-11 (00573055211)
| NDC Package Code | 0573-0552-11 |
|---|---|
| Billing NDC | 00573055211 |
| Package | 1 TUBE in 1 CARTON (0573-0552-11) / 26 g in 1 TUBE |
| Marketing Start Date | 2020-02-28 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL d6148d77-ef6f-4552-b5ba-43b429923f19 Details
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
Warnings
For external use only
When using this product
- •
- avoid contact with the eyes
- •
- do not exceed the recommended daily dosage unless directed by a doctor
- •
- do not put into the rectum by using fingers or any mechanical device or applicator
SPL UNCLASSIFIED SECTION
Directions
- •
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or soft cloth before application of this product.
- •
- when first opening the tube, puncture foil seal with top end of cap
- •
- adults and children 12 years of age and older: apply to the affected area not more than 3 to 4 times daily
- •
- children under 12 years of age: do not use, consult a doctor
SPL UNCLASSIFIED SECTION
Inactive ingredients
anhydrous citric acid, butylated hydroxyanisole, carboxymethylcellulose sodium, cetyl alcohol, citric acid monohydrate, edetate disodium, glycerin, glyceryl oleate, glyceryl stearate, lanolin, methylparaben, propyl gallate, propylene glycol, propylparaben, purified water, simethicone emulsion, sodium benzoate, sodium lauryl sulfate, stearyl alcohol, white petrolatum, xanthan gum
SPL UNCLASSIFIED SECTION
PRINCIPAL DISPLAY PANEL - 26 g Tube Label
PRINCIPAL DISPLAY PANEL - 26 g Tube Carton
INGREDIENTS AND APPEARANCE
| PREPARATION H SOOTHING RELIEF ANTI-ITCH
hydrocortisone cream |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |