Search by Drug Name or NDC
NDC 00574-7034-12 aspirin 300 mg/1 Details
aspirin 300 mg/1
aspirin is a RECTAL SUPPOSITORY in the HUMAN OTC DRUG category. It is labeled and distributed by Padagis US LLC. The primary component is ASPIRIN.
MedlinePlus Drug Summary
Aspirin rectal is used to reduce fever and to relieve mild to moderate pain from headaches, menstrual periods, arthritis, toothaches, and muscle aches. Aspirin is in a group of medications called salicylates. It works by stopping the production of certain natural substances that cause fever, pain, swelling, and blood clots.
Related Packages: 00574-7034-12Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Aspirin Rectal
Product Information
| NDC | 00574-7034 |
|---|---|
| Product ID | 0574-7034_1fde5a34-6494-43b5-a12a-c3ef3964d882 |
| Associated GPIs | 64100010005218 |
| GCN Sequence Number | 004371 |
| GCN Sequence Number Description | aspirin SUPP.RECT 300 MG RECTAL |
| HIC3 | H3D |
| HIC3 Description | ANALGESIC/ANTIPYRETICS, SALICYLATES |
| GCN | 16696 |
| HICL Sequence Number | 001820 |
| HICL Sequence Number Description | ASPIRIN |
| Brand/Generic | Generic |
| Proprietary Name | aspirin |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | aspirin |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | SUPPOSITORY |
| Route | RECTAL |
| Active Ingredient Strength | 300 |
| Active Ingredient Units | mg/1 |
| Substance Name | ASPIRIN |
| Labeler Name | Padagis US LLC |
| Pharmaceutical Class | Anti-Inflammatory Agents, Non-Steroidal [CS], Cyclooxygenase Inhibitors [MoA], Decreased Platelet Aggregation [PE], Decreased Prostaglandin Production [PE], Nonsteroidal Anti-inflammatory Drug [EPC], Platelet Aggregation Inhibitor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

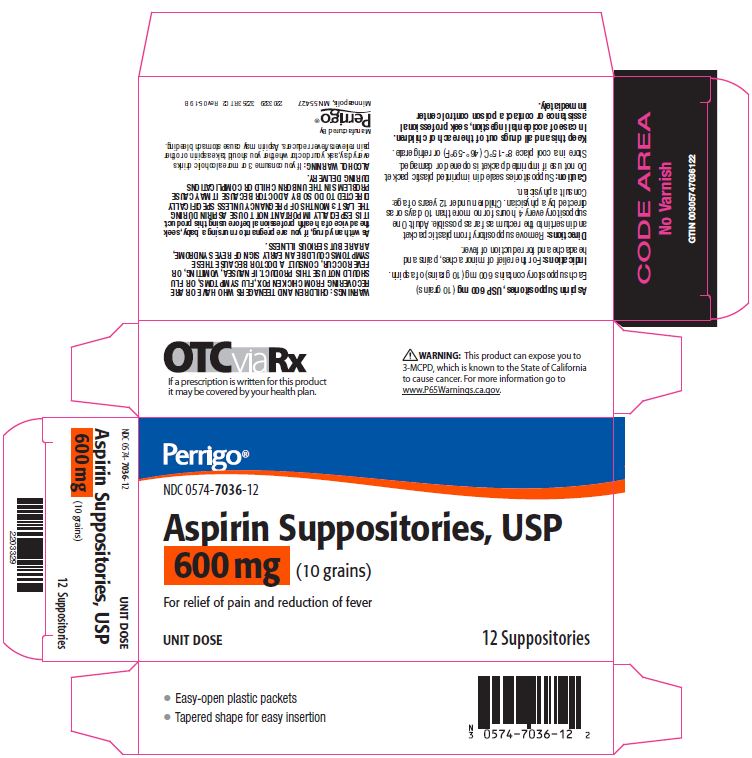
NDC 00574-7034-12 (00574703412)
| NDC Package Code | 0574-7034-12 |
|---|---|
| Billing NDC | 00574703412 |
| Package | 12 PACKET in 1 CARTON (0574-7034-12) / 1 SUPPOSITORY in 1 PACKET |
| Marketing Start Date | 1990-09-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL cce244aa-cd79-49c8-b68e-267f2bd0ef67 Details
Active ingredient
Directions
Caution:
Keep this and all drugs out of the reach of children.
Warnings
CHILDREN AND TEENAGERS WHO HAVE OR ARE RECOVERING FROM CHICKEN POX, FLU SYMPTOMS, OR FLU SHOULD NOT USE THIS PRODUCT. IF NAUSEA, VOMITING, OR FEVER OCCUR, CONSULT A DOCTOR BECAUSE THESE SYMPTOMS COULD BE AN EARLY SIGN OF REYE SYNDROME, A RARE BUT SERIOUS ILLNESS.
As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product. IT IS ESPECIALLY IMPORTANT NOT TO USE ASPIRIN DURING THE LAST 3 MONTHS OF PREGNANCY UNLESS SPECIFICALLY DIRECTED TO DO SO BY A DOCTOR BECAUSE IT MAY CAUSE PROBLEMS IN THE UNBORN CHILD OR COMPLICATIONS DURING DELIVERY.
ALCOHOL WARNING:
Package/Label Principal Display Panel – 300 mg
Package/Label Principal Display Panel – 600 mg
INGREDIENTS AND APPEARANCE
| ASPIRIN
aspirin suppository |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ASPIRIN
aspirin suppository |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Padagis US LLC (967694121) |