Search by Drug Name or NDC
NDC 13985-0607-03 Neomycin and Polymyxin B Sulfates, and Bacitracin Zinc 400; 3.5; 10000 [USP'U]/g; mg/g; [USP'U]/g Details
Neomycin and Polymyxin B Sulfates, and Bacitracin Zinc 400; 3.5; 10000 [USP'U]/g; mg/g; [USP'U]/g
Neomycin and Polymyxin B Sulfates, and Bacitracin Zinc is a OPHTHALMIC OINTMENT in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by MWI. The primary component is BACITRACIN ZINC; NEOMYCIN SULFATE; POLYMYXIN B SULFATE.
MedlinePlus Drug Summary
Neomycin, polymyxin, and bacitracin ophthalmic combination is used to treat eye and eyelid infections. Neomycin, polymyxin, and bacitracin are in a class of medications called antibiotics. Neomycin, polymyxin, and bacitracin combination works by stopping the growth of bacteria infecting a surface of the eye.
Related Packages: 13985-0607-03Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Neomycin, Polymyxin, and Bacitracin Ophthalmic
Product Information
| NDC | 13985-0607 |
|---|---|
| Product ID | 13985-607_95de95d4-4393-4a34-a9c7-63169e112f85 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Neomycin and Polymyxin B Sulfates, and Bacitracin Zinc |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Neomycin Sulfate, Polymyxin B Sulfate and Bacitracin Zinc |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | OINTMENT |
| Route | OPHTHALMIC |
| Active Ingredient Strength | 400; 3.5; 10000 |
| Active Ingredient Units | [USP'U]/g; mg/g; [USP'U]/g |
| Substance Name | BACITRACIN ZINC; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Labeler Name | MWI |
| Pharmaceutical Class | Aminoglycoside Antibacterial [EPC], Aminoglycosides [CS], Decreased Cell Wall Synthesis & Repair [PE], Polymyxin-class Antibacterial [EPC], Polymyxins [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA065088 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images


NDC 13985-0607-03 (13985060703)
| NDC Package Code | 13985-607-03 |
|---|---|
| Billing NDC | 13985060703 |
| Package | 1 TUBE in 1 CARTON (13985-607-03) / 3.5 g in 1 TUBE |
| Marketing Start Date | 2015-03-23 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 0f651b47-ef7e-4d07-b3b1-8baade8b9031 Details
DESCRIPTION:
Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, USP is a sterile antimicrobial ointment for ophthalmic use. Each gram contains: Neomycin Sulfate (equivalent to 3.5 mg neomycin base), Polymyxin B sulfate equal to 10,000 polymyxin B units, bacitracin zinc equal to 400 bacitracin units, and white petrolatum, q.s.
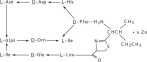
Neomycin sulfate is the sulfate salt of neomycin B and C, which are produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetaceae). It has a potency equivalent of not less than 600 mcg of neomycin standard per mg, calculated on an anhydrous basis. The structural formula are:
Polymyxin B sulfate is the sulfate salt of polymyxin B1 and B2, which are produced by the growth of Bacillus palymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per mg, calculated on an anhydrous basis. The structural formulae are:
Bacitracin zinc is the zinc salt of bacitracin, a mixture of related cyclic polypeptides (mainly bacitracin A) produced by the growth of an organism of the lichenifoimis group of Bacillus subtilis var Tracy. It has a potency of not less than 40 bacitracin units per mg. The structural formula is:
CLINICAL PHARMACOLOGY:
A wide range of antibacterial action is provided by the overlapping spectra of neomycin, polymyxin B sulfate, and bacitracin.
Neomycin is bactericidal for many gram-positive and gram-negative organisms. It is an aminoglycoside antibiotic, which inhibits protein synthesis by binding with ribosomal RNA and causing misreading of the bacterial genetic code.
Polymyxin B is bactericidal for a variety of gram-negative organisms. It increases the permeability of the bacterial cell membrane by interacting with the phospholipid components of the membrane.
Bacitracin is bactericidal for a variety of gram-positive and gram-negative organisms. It interferes with bacterial cell wall synthesis by inhibition of the regeneration of phospholipid receptors involved in phospholipid synthesis.
Microbiology: Neomycin sulfate, polymyxin B sulfate, and bacitracin zinc together are considered active against the following microorganisms: Staphylococcus aureus, streptococci including Streptococcus pneumoniae, Escherichiacoli, Haemophilusinfluenzae, Klebsiella/Enterobacter species, Neisseria species, and Pseudomonas aeruginosa. The product does not provide adequate coverage against Serratia marcescens.
INDICATIONS AND USAGE:
Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, USP is indicated for the topical treatment of superficial infections of the external eye and its adnexa caused by susceptible bacteria. Such infections encompass conjunctivitis, keratitis and keratoconjunctivitis, blepharitis and blepharoconjunctivitis.
CONTRAINDICATIONS:
WARNINGS:
NOT FOR INJECTION INTO THE EYE. Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ointment USP should never be directly introduced into the anterior chamber of the eye. Ophthalmic ointments may retard corneal wound healing.
Topical antibiotics, particularly neomycin sulfate, may cause cutaneous sensitization. A precise incidence of hypersensitivity reactions (primarily skin rash) due to topical antibiotics is not known. The manifestations of sensitization to topical antibiotics are usually itching, reddening, and edema of the conjunctiva and eyelid.
A sensitization reaction may manifest simply as a failure to heal. During long-term use of topical antibiotic products, periodic examination for such signs is advisable, and the patient should be told to discontinue the product if they are observed. Symptoms usually subside quickly on withdrawing the medication. Application of products containing these ingredients should be avoided for the patient thereafter (see PRECAUTIONS: General).
PRECAUTIONS:
General: As with other antibiotic preparations, prolonged use of Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment USP may result in overgrowth of nonsusceptible organisms including fungi. If superinfection occurs, appropriate measures should be initiated.
Bacterial resistance to Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment USP may also develop. If purulent discharge, inflammation, or pain becomes aggravated, the patient should discontinue use of the medication and consult a physician.
There have been reports of bacterial keratitis associated with the use of topical ophthalmic products in multiple-dose containers, which have been inadvertently contaminated by patients, most of whom had a concurrent corneal disease or a disruption of the ocular epithelial surface (see PRECAUTIONS: Information for Patients).
Allergic cross-reactions may occur which could prevent the use of any or all of the following antibiotics for the treatment of future infections: kanamycin, paromomycin, streptomycin, and possibly gentamicin.
Information for Patients: Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye, eyelid, fingers, or any other surface. The use of this product by more than one person may spread infection.
Patients should also be instructed that ocular products, if handled improperly, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated products (see PRECAUTIONS: General).
If the condition persists or gets worse, or if a rash or allergic reaction develops, the patient should be advised to stop use and consult a physician. Do not use this product if you are allergic to any of the listed ingredients.
Keep tightly closed when not in use. Keep out of reach of children.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies in animals to evaluate carcinogenic or mutagenic potential have not been conducted with polymyxin B sulfate or bacitracin. Treatment of cultured human lymphocytes in vitro with neomycin increased the frequency of chromosome aberrations at the highest concentration (80 mcg/mL) tested; however, the effects of neomycin on carcinogenesis and mutagenesis in humans are unknown.
Polymyxin B has been reported to impair the motility of equine sperm, but its effects on male or female fertility are unknown. No adverse effects on male or female fertility, liter size or survival were observed in rabbits given bacitracin zinc 100 gm/ton of diet.
Pregnancy: Teratogenic Effects: Pregnancy Category C. Animal reproduction studies have not been conducted with neomycin sulfate, polymyxin B sulfate, or bacitracin. It is also not known whether Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, USP should be given to pregnant woman only if dearly needed.
ADVERSE REACTIONS:
Adverse reactions have occurred with the anti-infective components of Neomycin and Polymyxin B Sulfate and Bacitracin Zinc Ophthalmic Ointment USP. The exact incidence is not known. Reactions occurring most often are allergic sensitization reactions including itching, swelling, and conjunctival erythema (see WARNINGS). More serious hypersensitivity reactions, including anaphylaxis, have been reported rarely.
Local irritation on instillation has also been reported.
DOSAGE AND ADMINISTRATION:
HOW SUPPLIED:
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| NEOMYCIN AND POLYMYXIN B SULFATES, AND BACITRACIN ZINC
neomycin sulfate, polymyxin b sulfate and bacitracin zinc ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - MWI (019926120) |
| Registrant - Akorn Operating Company LLC (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696840 | MANUFACTURE(13985-607) , ANALYSIS(13985-607) , STERILIZE(13985-607) , LABEL(13985-607) , PACK(13985-607) | |