Search by Drug Name or NDC
NDC 16571-0694-01 CARBIDOPA, LEVODOPA AND ENTACAPONE 50; 200; 200 mg/1; mg/1; mg/1 Details
CARBIDOPA, LEVODOPA AND ENTACAPONE 50; 200; 200 mg/1; mg/1; mg/1
CARBIDOPA, LEVODOPA AND ENTACAPONE is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Rising Pharma Holdings, Inc.. The primary component is CARBIDOPA; ENTACAPONE; LEVODOPA.
MedlinePlus Drug Summary
Entacapone is an inhibitor of catechol-O-methyltransferase (COMT). It is used in combination with levodopa and carbidopa (Sinemet) to treat the end-of-dose 'wearing-off' symptoms of Parkinson's disease. Entacapone helps the levodopa and carbidopa work better by allowing more of it to reach the brain, where it has its effects. This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
Related Packages: 16571-0694-01Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Entacapone
The combination of levodopa and carbidopa is used to treat the symptoms of Parkinson's disease and Parkinson's-like symptoms that may develop after encephalitis (swelling of the brain) or injury to the nervous system caused by carbon monoxide poisoning or manganese poisoning. Parkinson's symptoms, including tremors (shaking), stiffness, and slowness of movement, are caused by a lack of dopamine, a natural substance usually found in the brain. Levodopa is in a class of medications called central nervous system agents. It works by being converted to dopamine in the brain. Carbidopa is in a class of medications called decarboxylase inhibitors. It works by preventing levodopa from being broken down before it reaches the brain. This allows for a lower dose of levodopa, which causes less nausea and vomiting.
Related Packages: 16571-0694-01Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Levodopa and Carbidopa
Product Information
| NDC | 16571-0694 |
|---|---|
| Product ID | 16571-694_bb341ca1-04d3-4a71-b79d-824653aa58a3 |
| Associated GPIs | 73209903300350 |
| GCN Sequence Number | 063191 |
| GCN Sequence Number Description | carbidopa/levodopa/entacapone TABLET 50-200-200 ORAL |
| HIC3 | H6A |
| HIC3 Description | ANTIPARKINSONISM DRUGS,OTHER |
| GCN | 98948 |
| HICL Sequence Number | 025476 |
| HICL Sequence Number Description | CARBIDOPA/LEVODOPA/ENTACAPONE |
| Brand/Generic | Generic |
| Proprietary Name | CARBIDOPA, LEVODOPA AND ENTACAPONE |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | CARBIDOPA, LEVODOPA AND ENTACAPONE |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 50; 200; 200 |
| Active Ingredient Units | mg/1; mg/1; mg/1 |
| Substance Name | CARBIDOPA; ENTACAPONE; LEVODOPA |
| Labeler Name | Rising Pharma Holdings, Inc. |
| Pharmaceutical Class | Amino Acids, Aromatic [CS], Aromatic Amino Acid [EPC], Catechol O-Methyltransferase Inhibitors [MoA], Catechol-O-Methyltransferase Inhibitor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA213212 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images





NDC 16571-0694-01 (16571069401)
| NDC Package Code | 16571-694-01 |
|---|---|
| Billing NDC | 16571069401 |
| Package | 100 TABLET, FILM COATED in 1 BOTTLE (16571-694-01) |
| Marketing Start Date | 2021-08-05 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.89983 |
| Pricing Unit | EA |
| Effective Date | 2024-01-17 |
| NDC Description | CARBIDOPA-LEVODOPA-ENTACAPONE 50-200-200 MG TAB |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 4 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 2f8e82d7-6baf-456a-b8c3-4fda6e317902 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
CARBIDOPA, LEVODOPA AND ENTACAPONE tablets, for oral use
Initial U.S. Approval: 2003
INDICATIONS AND USAGE
Carbidopa, levodopa and entacapone tablets, a combination drug consisting of levodopa (aromatic amino acid), carbidopa (aromatic amino acid decarboxylation inhibitor), and entacapone (catechol-O-methyltransferase (COMT) inhibitor) is indicated for the treatment of Parkinson’s disease.
Carbidopa, levodopa and entacapone tablets are to be used:
- To substitute (with equivalent strengths of each of the three components) for carbidopa/levodopa and entacapone previously administered as individual products (1)
- To replace carbidopa/levodopa therapy (without entacapone) when patients experience the signs and symptoms of end-of-dose “wearing-off” and when they have been taking a total daily dose of levodopa of 600 mg or less and have not been experiencing dyskinesias (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
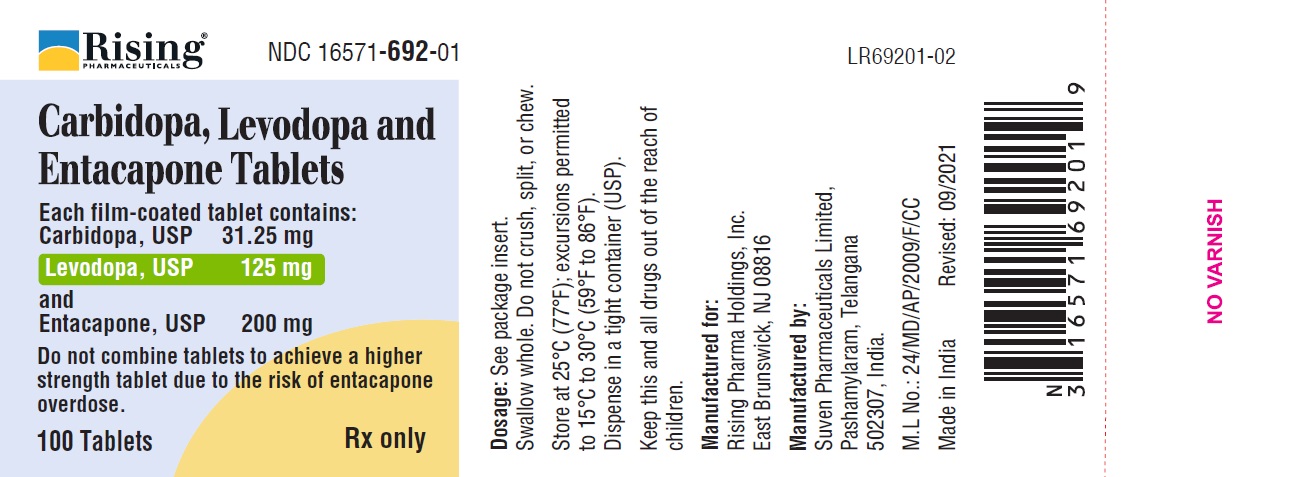
Each carbidopa, levodopa and entacapone tablet contains a 1:4 ratio of carbidopa to levodopa and 200 mg of entacapone (mg of carbidopa per mg of levodopa per mg of entacapone) (3)
- 12.5 mg per 50 mg per 200 mg
- 18.75 mg per 75 mg per 200 mg
- 25 mg per 100 mg per 200 mg
- 31.25 mg per 125 mg per 200 mg
- 37.5 mg per 150 mg per 200 mg
- 50 mg per 200 mg per 200 mg
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- May cause falling asleep during activities of daily living without apparent warning, and daytime drowsiness and somnolence (5.1)
- May cause syncope and hypotension/orthostatic hypotension (5.2)
- May cause or exacerbate dyskinesia (5.3)
- May cause depression and suicidality (5.4)
- May cause hallucinations and/or other psychotic-like behavior (5.5)
- May cause problems with impulse control and compulsive behaviors (5.6)
- Abrupt discontinuation may cause hyperpyrexia and confusion (5.7)
- May cause diarrhea and/or drug-induced colitis (5.8)
- May cause rhabdomyolysis (5.9)
ADVERSE REACTIONS
The most common adverse reactions (incidence 3% higher than placebo incidence) are dyskinesias, hyperkinesia, diarrhea, nausea, abdominal pain, vomiting, dry mouth, and urine discoloration (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharma Holdings, Inc. at 1-844-874-7464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Drugs metabolized by COMT: use with caution (5.10, 7.2)
- Anti-hypertensive agents: dose adjustment may be required (7.3)
- Tricyclic antidepressants: risk of hypertension and dyskinesia reported during concomitant use with carbidopa/levodopa (7.4)
- Dopamine D2 receptor antagonists, isoniazid, phenytoin, papaverine and iron salts: may reduce efficacy of carbidopa, levodopa and entacapone tablets (7.5, 7.6, 7.7, 7.8, 7.9)
- Drugs that interfere with biliary excretion, glucuronidation and intestinal beta-glucuronidase: dose adjustment of carbidopa, levodopa and entacapone tablets may be required (7.10)
- Drugs metabolized by CYP2C9 (e.g., coumadin): dose adjustment of carbidopa, levodopa and entacapone tablets may be required; monitor INR when initiating carbidopa, levodopa and entacapone tablets in patients on coumadin (7.11)
USE IN SPECIFIC POPULATIONS
- Pregnancy: based on animal data, may cause fetal harm (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Converting Patients from Carbidopa, Levodopa, and Entacapone to Carbidopa, Levodopa and Entacapone Tablets
2.3 Converting Patients from Carbidopa and Levodopa Products to Carbidopa, Levodopa and Entacapone Tablets
2.4 Concomitant Use with Other Anti-Parkinson’s Disease Drugs
2.5 Decrease or Interruption of Dosing
2.6 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
5.2 Hypotension, Orthostatic Hypotension and Syncope
5.3 Dyskinesia
5.4 Depression and Suicidality
5.5 Hallucinations and/or Psychotic-Like Behavior
5.6 Impulse Control and/or Compulsive Behaviors
5.7 Withdrawal-Emergent Hyperpyrexia and Confusion
5.8 Diarrhea and Colitis
5.9 Rhabdomyolysis
5.10 Interaction with Drugs Metabolized by COMT
5.11 Fibrotic Complications
5.12 Peptic Ulcer Disease
5.13 Hepatic Impairment
5.14 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 MAO Inhibitors
7.2 Drugs Metabolized by Catechol-O-Methyltransferase (COMT)
7.3 Antihypertensive Agents
7.4 Tricyclic Antidepressants
7.5 Dopamine D2 Receptor Antagonists
7.6 Isoniazid
7.7 Phenytoin
7.8 Papaverine
7.9 Iron Salts
7.10 Drugs Known to Interfere with Biliary Excretion, Glucuronidation, and Intestinal Beta-glucuronidase
7.11 Drugs Metabolized via CYP2C9 (e.g., coumadin)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment or Biliary Obstruction
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdosage
10.2 Management of Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.6 Hormone Levels
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
Carbidopa, levodopa and entacapone tablets, are indicated for the treatment of Parkinson’s disease.
Carbidopa, levodopa and entacapone tablets can be used:
- To substitute (with equivalent strengths of each of the three components) carbidopa/levodopa and entacapone previously administered as individual products.
- To replace carbidopa/levodopa therapy (without entacapone) when patients experience the signs and symptoms of end-of-dose “wearing-off” and when they have been taking a total daily dose of levodopa of 600 mg or less and have not been experiencing dyskinesias.
2 DOSAGE AND ADMINISTRATION
Carbidopa, levodopa and entacapone tablets should be used as a substitute for patients already stabilized on equivalent doses of carbidopa/levodopa and entacapone. However, some patients who have been stabilized on a given dose of carbidopa/levodopa may be treated with carbidopa, levodopa and entacapone tablets if a decision has been made to add entacapone (see below). Therapy should be individualized and adjusted according to the desired therapeutic response.
2.1 Dosing Information
The optimum daily dosage of carbidopa, levodopa and entacapone tablets must be determined by careful titration in each patient.
Clinical experience with daily doses above 1,600 mg of entacapone is limited. The maximum recommended daily dose of carbidopa, levodopa and entacapone tablets depends on the strength used. The maximum number of tablets to be used in a 24-hour period is less with the highest strength (carbidopa, levodopa and entacapone tablets 50 mg/200 mg/200 mg) than with lower strengths (see Table 1). Studies show that peripheral dopa decarboxylase is saturated by carbidopa at approximately 70 mg per day to 100 mg per day. Patients receiving less than this amount of carbidopa are more likely to experience nausea and vomiting.
| Carbidopa, Levodopa and Entacapone Tablets Dosage Strength | Maximum Number of Tablets in a 24-hour Period |
| 12.5 mg per 50 mg per 200 mg, 18.75 mg per 75 mg per 200 mg, 25 mg per 100 mg per 200 mg, 31.25 mg per 125 mg per 200 mg, 37.5 mg per 150 mg per 200 mg | 8 |
| 50 mg per 200 mg per 200 mg |
6 |
2.2 Converting Patients from Carbidopa, Levodopa, and Entacapone to Carbidopa, Levodopa and Entacapone Tablets
Patients currently treated with entacapone 200 mg with each dose of non-extended release carbidopa/levodopa tablet, can switch to the corresponding strength of carbidopa, levodopa and entacapone tablets containing the same amounts of levodopa and carbidopa. For example, patients receiving one tablet of carbidopa/levodopa 25 mg/100 mg and one tablet of entacapone 200 mg at each administration can switch to a single carbidopa, levodopa and entacapone 25 mg/100 mg/200 mg tablet (containing 25 mg of carbidopa, 100 mg of levodopa and 200 mg of entacapone).
2.3 Converting Patients from Carbidopa and Levodopa Products to Carbidopa, Levodopa and Entacapone Tablets
There is no experience in transferring patients currently treated with extended release formulations of carbidopa/levodopa, or carbidopa/levodopa products that are not combined in a 1:4 ratio of carbidopa to levodopa.
Patients with a history of moderate or severe dyskinesias or taking more than 600 mg of the levodopa component per day are likely to require a reduction in their daily levodopa dose when entacapone is added. Because dose adjustment of the individual carbidopa or levodopa component is not possible with fixed-dose products, initially titrate patients to a dose that is tolerated and that meets their individual therapeutic need using a separate carbidopa/levodopa tablet (1:4 ratio) plus an entacapone tablet. Once the patient’s individual dose of carbidopa/levodopa plus entacapone dose has been established using two separate tablets; switch the patient to a corresponding single tablet of carbidopa, levodopa and entacapone.
When less levodopa is required, reduce the total daily dosage of carbidopa/levodopa either by decreasing the strength of carbidopa, levodopa and entacapone tablets at each administration or by decreasing the frequency of administration by extending the time between doses.
2.4 Concomitant Use with Other Anti-Parkinson’s Disease Drugs
Anticholinergic agents, dopamine agonists, monoamine oxidase (MAO) - B inhibitors, amantadine, and other standard drugs for Parkinson's disease may be used concomitantly while carbidopa, levodopa and entacapone tablets is being administered; however, dosage adjustments of the concomitant medication or carbidopa, levodopa and entacapone tablets may be required.
2.5 Decrease or Interruption of Dosing
Avoid interruption of carbidopa, levodopa and entacapone tablets dosing because hyperpyrexia has been reported in patients who suddenly discontinue or reduce their use of levodopa [see Warnings and Precautions (5.7)].
2.6 Important Administration Instructions
Do not split, crush or chew carbidopa, levodopa and entacapone tablets. Administer only one tablet at each dosing interval. All strengths of carbidopa, levodopa and entacapone tablets contain 200 mg of entacapone. Combining multiple tablets or portions of tablets to achieve a higher levodopa dose may lead to an overdose of entacapone.
Administer carbidopa, levodopa and entacapone tablets with or without food. However, a high-fat, high-calorie meal may delay the absorption of levodopa by about 2 hours [see Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
Each carbidopa, levodopa and entacapone tablet, provided in 6 single-dose strengths, contains carbidopa and levodopa in a 1:4 ratio and a 200 mg dose of entacapone. Carbidopa, levodopa and entacapone tablets are supplied as film-coated tablets for oral administration in the following 6 strengths:
Carbidopa, levodopa and entacapone film-coated tablets 12.5 mg/50mg/ 200mg containing 12.5 mg of carbidopa, 50 mg of levodopa and 200 mg of entacapone. The unscored, round, biconvex shaped tablets are brownish and debossed “S 50” on one side and plain on other side.
Carbidopa, levodopa and entacapone film-coated tablets 18.75 mg/75 mg/200 mg containing 18.75 mg of carbidopa, 75 mg of levodopa and 200 mg of entacapone. The unscored, oval, biconvex shaped tablets are light brownish and debossed “S75” on one side and plain on other side.
Carbidopa, levodopa and entacapone film-coated tablets 25 mg/100 mg/200 mg containing 25 mg of carbidopa, 100 mg of levodopa and 200 mg of entacapone. The unscored, oval, biconvex shaped tablets are brownish and debossed “S100” on one side and plain on other side.
Carbidopa, levodopa and entacapone film-coated tablets 31.25 mg/125 mg/200 mg containing 31.25 mg of carbidopa, 125 mg of levodopa and 200 mg of entacapone. The unscored, oval, biconvex shaped tablets are light brownish and debossed “S125” on one side and plain on other side.
Carbidopa, levodopa and entacapone film-coated tablets 37.5 mg/150 mg/200 mg containing 37.5 mg of carbidopa, 150 mg of levodopa and 200 mg of entacapone. The unscored, elongated ellipse shaped tablets are brownish and debossed “S150” on one side and plain on other side.
Carbidopa, levodopa and entacapone film-coated tablets 50 mg/200 mg/200 mg containing 50 mg of carbidopa, 200 mg of levodopa and 200 mg of entacapone. The unscored, oval shaped tablets are brownish red and debossed “S200” on one side and plain on other side.
4 CONTRAINDICATIONS
Carbidopa, levodopa and entacapone tablets are contraindicated in patients:
- Taking nonselective monoamine oxidase (MAO) inhibitors (e.g., phenelzine and tranylcypromine). These nonselective MAO inhibitors must be discontinued at least two weeks prior to initiating therapy with carbidopa, levodopa and entacapone tablets.
- With narrow-angle glaucoma.
5 WARNINGS AND PRECAUTIONS
The following adverse reactions described in this section are related to at least one of the components of carbidopa, levodopa and entacapone tablets (i.e., levodopa, carbidopa, and/or entacapone) based upon the safety experience in clinical trials (especially pivotal trials) or in postmarketing reports.
5.1 Falling Asleep During Activities of Daily Living and Somnolence
Patients with Parkinson’s disease treated with carbidopa, levodopa and entacapone tablets or other carbidopa/levodopa products have reported suddenly falling asleep without prior warning of sleepiness while engaged in activities of daily living (including the operation of motor vehicles). Some of these episodes resulted in accidents. Although many of these patients reported somnolence while taking entacapone, some did not perceive warning signs, such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events have been reported to occur up to one year after initiation of treatment.
Somnolence was reported in 2% of patients taking entacapone and 0% in placebo in controlled trials. It is reported that falling asleep while engaged in activities of daily living always occurs in a setting of pre-existing somnolence, although patients may not give such a history. For this reason, prescribers should reassess patients for drowsiness or sleepiness especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. Patients who have already experienced somnolence and/or an episode of sudden sleep onset should not participate in these activities during treatment with carbidopa, levodopa and entacapone tablets.
Before initiating treatment with carbidopa, levodopa and entacapone tablets, advise patients of the potential to develop drowsiness and specifically ask about factors that may increase this risk such as use of concomitant sedating medications and the presence of sleep disorders. If a patient develops daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.), carbidopa, levodopa and entacapone tablets should ordinarily be discontinued [see Dosage and Administration (2.5) and Warnings and Precautions (5.7)]. If the decision is made to continue carbidopa, levodopa and entacapone tablets, patients should be advised not to drive and to avoid other potentially dangerous activities. There is insufficient information to establish whether dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
5.2 Hypotension, Orthostatic Hypotension and Syncope
Reports of syncope were generally more frequent in patients in both treatment groups who had had a prior episode of documented hypotension (although the episodes of syncope, obtained by history, were themselves not documented with vital sign measurement). Hypotension, orthostatic hypotension, and syncope are observed in patients treated with drugs that increase central dopaminergic tone including carbidopa, levodopa and entacapone tablets.
5.3 Dyskinesia
Dyskinesia (involuntary movements) may occur or be exacerbated at lower dosages and sooner with carbidopa, levodopa and entacapone tablets than with preparations containing only carbidopa and levodopa. The occurrence of dyskinesias may require dosage reduction.
In pivotal trials, the treatment difference incidence of dyskinesia was 10% and for carbidopa-levodopa plus 200 mg entacapone. Although decreasing the dose of levodopa may ameliorate this side effect, many patients in controlled trials continued to experience frequent dyskinesias despite a reduction in their dose of levodopa. The treatment difference incidence of study withdrawal for dyskinesia was 1% for carbidopa-levodopa-entacapone.
5.4 Depression and Suicidality
All patients should be observed carefully for the development of depression with concomitant suicidal tendencies. Patients with past or current psychoses should be treated with caution.
5.5 Hallucinations and/or Psychotic-Like Behavior
Dopaminergic therapy in patients with Parkinson’s disease has been associated with hallucinations. Hallucinations led to drug discontinuation and premature withdrawal from clinical trials in 0.8% and 0% of patients treated with carbidopa, levodopa, entacapone and carbidopa, levodopa, respectively. Hallucinations led to hospitalization in 1.0% and 0.3% of patients in the carbidopa, levodopa, entacapone and carbidopa, levodopa, groups, respectively. Agitation occurred in 1% of patients treated with carbidopa, levodopa, entacapone and 0% treated with carbidopa, levodopa.
5.6 Impulse Control and/or Compulsive Behaviors
Postmarketing reports suggest that patients treated with anti-Parkinson medications can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, and other intense urges. Patients may be unable to control these urges while taking one or more of the medications generally used for the treatment of Parkinson’s disease and which increase central dopaminergic tone, including entacapone taken with levodopa and carbidopa. In some cases, although not all, these urges were reported to have stopped when the dose of anti-Parkinson medications was reduced or discontinued. Because patients may not recognize these behaviors as abnormal it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with entacapone. Physicians should consider dose reduction or stopping carbidopa, levodopa and entacapone tablets if a patient develops such urges while taking carbidopa, levodopa and entacapone tablets [see Dosage and Administration (2.5), Warnings and Precautions (5.7)].
5.7 Withdrawal-Emergent Hyperpyrexia and Confusion
Cases of hyperpyrexia and confusion resembling neuroleptic malignant syndrome (NMS) have been reported in association with dose reduction or withdrawal of therapy with carbidopa, levodopa and entacapone. However, in some cases, hyperpyrexia and confusion were reported after initiation of treatment with entacapone. Hyperpyrexia and confusion are uncommon but they may be life-threatening with a variety of features, including hyperpyrexia/fever/hyperthermia, muscle rigidity, involuntary movements, altered consciousness/mental status changes, delirium, autonomic dysfunction, tachycardia, tachypnea, sweating, hyper- or hypotension, and abnormal laboratory findings (e.g., creatine phosphokinase elevation, leukocytosis, myoglobinuria, and increased serum myoglobin).
If a patient needs to discontinue or reduce their daily dose of carbidopa, levodopa and entacapone tablets, the dose should be decreased slowly, with supervision from a health care provider [see Dosage and Administration (2.5)]. Specific methods for tapering entacapone have not been systematically evaluated.
5.8 Diarrhea and Colitis
In clinical trials of entacapone, diarrhea developed in 60 of 603 (10.0%) and 16 of 400 (4.0%) of patients treated with 200 mg of entacapone or placebo in combination with levodopa and dopa decarboxylase inhibitor, respectively. In patients treated with entacapone, diarrhea was generally mild to moderate in severity (8.6%) but was regarded as severe in 1.3%. Diarrhea resulted in withdrawal in 10 of 603 (1.7%) patients, 7 (1.2%) with mild and moderate diarrhea and 3 (0.5%) with severe diarrhea. Diarrhea generally resolved after discontinuation of entacapone. Two patients with diarrhea were hospitalized. Typically, diarrhea presents within 4 to 12 weeks after entacapone is started, but it may appear as early as the first week and as late as many months after the initiation of treatment. Diarrhea may be associated with weight loss, dehydration, and hypokalemia.
Postmarketing experience has shown that diarrhea may be a sign of drug-induced microscopic colitis, primarily lymphocytic colitis. In these cases diarrhea has usually been moderate to severe, watery and non-bloody, at times associated with dehydration, abdominal pain, weight loss, and hypokalemia. In the majority of cases, diarrhea and other colitis-related symptoms resolved or significantly improved when entacapone treatment was stopped. In some patients with biopsy confirmed colitis, diarrhea had resolved or significantly improved after discontinuation of entacapone but recurred after retreatment with entacapone.
If prolonged diarrhea is suspected to be related to carbidopa, levodopa and entacapone tablets, the drug should be discontinued and appropriate medical therapy considered. If the cause of prolonged diarrhea remains unclear or continues after stopping entacapone, then further diagnostic investigations including colonoscopy and biopsies should be considered.
5.9 Rhabdomyolysis
Cases of severe rhabdomyolysis have been reported with entacapone when used in combination with carbidopa and levodopa. Severe prolonged motor activity including dyskinesia may possibly account for rhabdomyolysis. Most of the cases were manifested by myalgia and increased values of creatine phosphokinase (CPK) and myoglobin. Some of the reactions also included fever and/or alteration of consciousness. It is also possible that rhabdomyolysis may be a result of the syndrome described in Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.7)].
5.10 Interaction with Drugs Metabolized by COMT
Drugs known to be metabolized by COMT, such as isoproterenol, epinephrine, norepinephrine, dopamine, dobutamine, alpha-methyldopa, apomorphine, isoetherine, and bitolterol should be administered with caution in patients receiving entacapone regardless of the route of administration (including inhalation), as their interaction may result in increased heart rate, arrhythmia, and/or increased blood pressure.
5.11 Fibrotic Complications
Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, and pleural thickening have been reported in some patients treated with ergot derived dopaminergic agents. These complications may resolve when the drug is discontinued, but complete resolution does not always occur. Although these adverse reactions may be related to the ergoline structure of these compounds, a possible causal role of nonergot derived drugs (e.g., entacapone, levodopa), which increase dopaminergic activity, has also been considered. The expected incidence of fibrotic complications is so low that even if entacapone caused these complications at rates similar to those attributable to other dopaminergic therapies, it is unlikely that it would have been detected in a cohort of the size exposed to entacapone during its clinical development. Four cases of pulmonary fibrosis have been reported during clinical development of entacapone; 3 of these patients were also treated with pergolide and 1 with bromocriptine. The duration of treatment with entacapone ranged from 7 months to 17 months.
5.12 Peptic Ulcer Disease
As with levodopa, treatment with carbidopa, levodopa and entacapone tablets may increase the possibility of upper gastrointestinal hemorrhage in patients with a history of peptic ulcer.
5.13 Hepatic Impairment
Patients with hepatic impairment should be treated with caution [see Clinical Pharmacology (12.3)]. As with levodopa, periodic evaluation of hepatic function is recommended during extended therapy.
5.14 Laboratory Tests
Abnormalities in laboratory tests may include elevations of liver function tests such as alkaline phosphatase, SGOT (AST), SGPT (ALT), lactic dehydrogenase, and bilirubin. Abnormalities in blood urea nitrogen and positive Coombs test have also been reported. Commonly, levels of blood urea nitrogen, creatinine, and uric acid are lower during administration of carbidopa, levodopa and entacapone tablets than with levodopa.
Carbidopa, levodopa and entacapone tablets may cause a false-positive reaction for urinary ketone bodies when a test tape is used for determination of ketonuria. This reaction will not be altered by boiling the urine specimen. False-negative tests may result with the use of glucose-oxidase methods of testing for glucosuria.
Cases of falsely diagnosed pheochromocytoma in patients on carbidopa/levodopa therapy have been reported very rarely. Caution should be exercised when interpreting the plasma and urine levels of catecholamines and their metabolites in patients on carbidopa/levodopa therapy.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in the Warnings and Precautions sections of labeling:
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.1)]
- Hypotension/Orthostatic Hypotension and Syncope [see Warnings and Precautions (5.2)]
- Dyskinesia [see Warnings and Precautions (5.3)]
- Depression and suicidality [see Warnings and Precautions (5.4)]
- Hallucinations/Psychotic-Like Behavior [see Warnings and Precautions (5.5)]
- Impulse Control and/or Compulsive Behaviors [see Warnings and Precautions (5.6)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.7)]
- Diarrhea and Colitis [see Warnings and Precautions (5.8)]
- Rhabdomyolysis [see Warnings and Precautions (5.9)]
- Peptic Ulcer Disease [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the incidence of adverse reactions (number of unique patients experiencing an adverse reaction associated with treatment/total number of patients treated) observed in the clinical trials of a drug cannot be directly compared to the incidence of adverse reactions in the clinical trials of another drug and may not reflect the incidence of adverse reactions observed in clinical practice.
Entacapone
The most commonly observed adverse reactions (incidence at least 3% greater than placebo incidence) in the double-blind, carbidopa-levodopa-placebo-controlled trials of entacapone (N=1,003 patients) associated with the use of carbidopa-levodopa-entacapone alone and not seen at an equivalent frequency among the placebo-treated patients were: dyskinesia, diarrhea, nausea, hyperkinesia, abdominal pain, vomiting, dry mouth, and urine discoloration.
The treatment difference incidence for premature study discontinuation for entacapone with levodopa and dopa decarboxylase inhibitor in the double-blind, placebo-controlled trials was 5%. The treatment difference incidence for the most frequent causes of study discontinuation was 2% for diarrhea, and 1% for other specific adverse reactions including psychiatric reasons, dyskinesia/ hyperkinesia, nausea, or abdominal pain.
Adverse Reaction Incidence in Controlled Clinical Studies of Entacapone
Table 2 lists treatment emergent adverse reactions that occurred in at least 1% of patients treated with carbidopa/levodopa and 200 mg of entacapone who participated in the double-blind, placebo-controlled studies, and that were numerically more common in this group than in the carbidopa/levodopa plus placebo group. In these studies, either entacapone or placebo was added to carbidopa/levodopa (or benserazide/levodopa).
| SYSTEM ORGAN CLASS Adverse Reaction | Carbidopa/levodopa plus Entacapone (n=603) % of patients | Carbidopa/levodopa plus Placebo (n=400) % of patients |
| SKIN AND APPENDAGES DISORDERS
|
||
| Sweating Increased | 2 | 1 |
| MUSCULOSKELETAL SYSTEM DISORDERS
|
||
| Back Pain | 5 | 3 |
| CENTRAL AND PERIPHERAL NERVOUS SYSTEM DISORDERS
|
||
| Dyskinesia | 25 | 15 |
| Hyperkinesia | 10 | 5 |
| Hypokinesia | 9 | 8 |
| Dizziness | 8 | 6 |
| SPECIAL SENSES, OTHER DISORDERS
|
||
| Taste Perversion | 1 | 0 |
| PSYCHIATRIC DISORDERS
|
||
| Anxiety | 2 | 1 |
| Somnolence | 2 | 0 |
| Agitation | 1 | 0 |
| GASTROINTESTINAL SYSTEM DISORDERS
|
||
| Nausea | 14 | 8 |
| Diarrhea | 10 | 4 |
| Abdominal Pain | 8 | 4 |
| Constipation | 6 | 4 |
| Vomiting | 4 | 1 |
| Mouth Dry | 3 | 0 |
| Dyspepsia | 2 | 1 |
| Flatulence | 2 | 0 |
| Gastritis | 1 | 0 |
| Gastrointestinal Disorders NOS | 1 | 0 |
| RESPIRATORY SYSTEM DISORDERS
|
||
| Dyspnea | 3 | 1 |
| PLATELET, BLEEDING AND CLOTTING DISORDERS
|
||
| Purpura | 2 | 1 |
| URINARY SYSTEM DISORDERS
|
||
| Urine Discoloration | 10 | 0 |
| BODY AS A WHOLE-GENERAL DISORDERS
|
||
| Fatigue | 6 | 4 |
| Asthenia | 2 | 1 |
| RESISTANCE MECHANISM DISORDERS
|
||
| Infection Bacterial | 1 | 0 |
6.2 Postmarketing Experience
The following spontaneous reports of adverse events temporally associated with entacapone or carbidopa, levodopa and entacapone tablets have been identified since market introduction and are not listed in Table 2. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish causal relationship to entacapone or carbidopa, levodopa and entacapone tablets exposure.
Hepatitis with mainly cholestatic features has been reported.
Effects of Gender and Age on Adverse Reactions
No differences were noted in the rate of adverse reactions attributable to entacapone alone by age or gender.
7 DRUG INTERACTIONS
7.1 MAO Inhibitors
Patients receiving nonselective MAO inhibitors and carbidopa, levodopa and entacapone may be at risk of increased adrenergic tone. Therefore, the use of carbidopa, levodopa and entacapone tablets is contraindicated in patients receiving nonselective MAO inhibitors [see Contraindications (4)].
7.2 Drugs Metabolized by Catechol-O-Methyltransferase (COMT)
Drugs known to be metabolized by COMT, such as isoproterenol, epinephrine, norepinephrine, dopamine, dobutamine, alpha-methyldopa, apomorphine, isoetherine, and bitolterol should be administered with caution in patients receiving entacapone regardless of the route of administration (including inhalation), as their interaction may result in increased heart rates, possibly arrhythmias, and excessive changes in blood pressure [see Warnings and Precautions (5.10)].
7.3 Antihypertensive Agents
Symptomatic postural hypotension has occurred when carbidopa/levodopa was added to the treatment of patients receiving antihypertensive drugs. When starting therapy with carbidopa, levodopa and entacapone tablets, dosage adjustment of antihypertensive drug may be required.
7.4 Tricyclic Antidepressants
There have been reports of adverse reactions, including hypertension and dyskinesia, resulting from the concomitant use of tricyclic antidepressants and carbidopa/levodopa.
7.5 Dopamine D2 Receptor Antagonists
Dopamine D2 receptor antagonists (e.g., metoclopramide, phenothiazines, butyrophenones, risperidone) may reduce the therapeutic effects of levodopa.
7.6 Isoniazid
Isoniazid may reduce the therapeutic effects of levodopa, a dose increase may be necessary.
7.7 Phenytoin
The beneficial effects of levodopa in Parkinson’s disease have been reported to be reversed by phenytoin. Patients taking phenytoin with carbidopa/levodopa should be carefully observed for loss of therapeutic response. Carbidopa, levodopa and entacapone tablets dosage should be increased as clinically needed in patients receiving phenytoin.
7.8 Papaverine
The beneficial effects of levodopa in Parkinson’s disease have been reported to be reversed by papaverine. Patients taking papaverine with carbidopa/levodopa should be carefully observed for loss of therapeutic response. Carbidopa, levodopa and entacapone tablets dosage should be increased as clinically needed in patients receiving papaverine.
7.9 Iron Salts
Iron salts or multi vitamins containing iron salts should be coadministered with caution. Iron salts can form chelates with levodopa, carbidopa and entacapone and consequently reduce bioavailability of levodopa, carbidopa and entacapone.
7.10 Drugs Known to Interfere with Biliary Excretion, Glucuronidation, and Intestinal Beta-glucuronidase
As most entacapone excretion is via the bile, caution should be exercised when drugs known to interfere with biliary excretion, glucuronidation, and intestinal beta-glucuronidase are given concurrently with entacapone. These include probenecid, cholestyramine, and some antibiotics (e.g., erythromycin, rifampicin, ampicillin and chloramphenicol).
7.11 Drugs Metabolized via CYP2C9 (e.g., coumadin)
The dosage of carbidopa, levodopa and entacapone tablets should be adjusted as clinically needed in patients using other drugs metabolized via CYP2C9. An interaction study in healthy volunteers, entacapone increased the AUC of R-warfarin on average by 18%, and the INR values on average by 13%. Cases of increased INR in patients concomitantly using warfarin have been reported during the post-approval use of entacapone. Thus, monitoring of INR is recommended when carbidopa, levodopa and entacapone tablets treatment is initiated for patients receiving warfarin.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of carbidopa, levodopa and entacapone tablets in pregnant women. In animals, administration of carbidopa-levodopa or entacapone during pregnancy was associated with developmental toxicity, including increased incidences of fetal malformations (see Data). The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2 to 4% and 15 to 20%, respectively.
Data
Animal data
In nonclinical studies in which carbidopa-levodopa was administered to pregnant animals, increased incidences of visceral and skeletal malformations were observed in rabbits at all doses and ratios of carbidopa-levodopa tested, which ranged from 10 times (carbidopa)-5 times (levodopa) to 20 times (carbidopa)-10 times (levodopa) the maximum recommended human dose (MRHD) of 1,600 mg/day. In rats, there was a decrease in the number of live pups delivered by dams receiving approximately two times (carbidopa)-five times (levodopa) the MRHD throughout organogenesis. No effects on malformation frequencies were observed in mice receiving up to 20 times the MRHD of carbidopa-levodopa.
In embryo-fetal development studies of entacapone, pregnant animals received doses of up to 1,000 mg/kg/day (rats) or 300 mg/kg/day (rabbits) throughout organogenesis. Increased incidences of fetal variations were evident in litters from rats treated with the highest dose, in the absence of overt signs of maternal toxicity. The maternal plasma entacapone exposure (AUC) associated with this dose was approximately 34 times that in humans at the MRHD. Increased frequencies of abortions and late/total resorptions and decreased fetal weights were observed in the litters of rabbits treated with maternally toxic doses of 100 mg/kg/day (plasma AUCs less than that in humans at the MRHD) or greater. There were no increases in malformation rates in these studies.
When entacapone was administered to female rats prior to mating and during early gestation, an increased incidence of fetal eye anomalies (macrophthalmia, microphthalmia, anophthalmia) was observed in the litters of dams treated with doses of 160 mg/kg/day (plasma AUCs seven times that in humans at the MRHD) or greater, in the absence of maternal toxicity. Administration of up to 700 mg/kg/day (plasma AUCs 28 times that in humans at the MRHD) to rats during the latter part of gestation and throughout lactation produced no evidence of developmental impairment in the offspring.
8.2 Lactation
Risk Summary
Levodopa has been detected in human milk after administration of carbidopa-levodopa. There are no data on the presence of entacapone or carbidopa in human milk, the effects of levodopa, carbidopa, or entacapone on the breastfed infant, or the effects on milk production. However, inhibition of lactation may occur because levodopa decreases secretion of prolactin [see Clinical Pharmacology (12.6)]. Carbidopa and entacapone are excreted in rat milk. In lactating rat, oral administration of radiolabeled entacapone resulted in levels of entacapone and/or metabolites in milk up to 2 to 3 times that in plasma.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for carbidopa, levodopa and entacapone tablets and any potential adverse effects on the breastfed infant from carbidopa, levodopa and entacapone tablets or from the underlying maternal condition.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of carbidopa, levodopa and entacapone tablets, 43.8% were 65 years old and over, while 7.2% were 75 years old and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients; however, greater sensitivity of some older individuals cannot be excluded.
Carbidopa, levodopa and entacapone tablets have not been studied in Parkinson’s disease patients or in healthy volunteers older than 75 years [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Renal impairment does not affect pharmacokinetics of entacapone. There are no studies on the pharmacokinetics of levodopa and carbidopa in patients with renal impairment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment or Biliary Obstruction
There are no studies on the pharmacokinetics of carbidopa and levodopa in patients with hepatic impairment. Carbidopa, levodopa and entacapone tablets should be administered cautiously to patients with biliary obstruction or hepatic disease since biliary excretion appears to be the major route of excretion of entacapone and hepatic impairment had a significant effect on the pharmacokinetics of entacapone when 200 mg entacapone was administered alone [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdosage
There are very few cases of overdose with levodopa reported in the published literature. Based on the available information, the acute symptoms of levodopa and dopa decarboxylase inhibitor overdose can be expected to arise from dopaminergic overstimulation. Doses of a few grams may result in CNS disturbances, with an increasing likelihood of cardiovascular disturbance (e.g., hypotension, tachycardia) and more severe psychiatric problems at higher doses. An isolated report of rhabdomyolysis and another of transient renal insufficiency suggest that levodopa overdose may give rise to systemic complications, secondary to dopaminergic overstimulation.
COMT inhibition by entacapone treatment is dose-dependent. A massive overdose of entacapone may theoretically produce a 100% inhibition of the COMT enzyme in people, thereby preventing the O-methylation of endogenous and exogenous catechols.
In clinical trials, the highest single dose of entacapone administered to humans was 800 mg, resulting in a plasma concentration of 14.1 mcg per mL. The highest daily dose given to humans was 2,400 mg, administered in one study as 400 mg six times daily with carbidopa/levodopa for 14 days in 15 Parkinson’s disease patients, and in another study as 800 mg three times daily for 7 days in 8 healthy volunteers. At this daily dose, the peak plasma concentrations of entacapone averaged 2.0 mcg per mL (at 45 min, compared to 1.0 mcg per mL and 1.2 mcg per mL with 200 mg entacapone at 45 min.). Abdominal pain and loose stools were the most commonly observed adverse events during this study. Daily doses as high as 2,000 mg entacapone have been administered as 200 mg 10 times daily with carbidopa/levodopa or benserazide/levodopa for at least 1 year in 10 patients, for at least 2 years in 8 patients and for at least 3 years in 7 patients. Overall, however, clinical experience with daily doses above 1,600 mg is limited.
10.2 Management of Overdosage
Hospitalization is advised, and general supportive measures should be employed, along with immediate gastric lavage and repeated doses of charcoal over time. This may hasten the elimination of entacapone in particular, by decreasing its absorption and reabsorption from the GI tract. Intravenous fluids should be administered judiciously and an adequate airway maintained.
Respiratory, circulatory and renal function should be monitored and appropriate supportive measures employed. Electrocardiographic monitoring should be instituted and the patient carefully observed for the development of arrhythmias; if required, appropriate antiarrhythmic therapy should be given. The possibility that the patient may have taken other drugs, increasing the risk of drug interactions (especially catechol-structured drugs) should be taken into consideration. To date, no experience has been reported with dialysis; hence, its value in overdosage is not known. Hemodialysis or hemoperfusion is unlikely to reduce entacapone levels due to its high binding to plasma proteins.
Pyridoxine is not effective in reversing the actions of carbidopa, levodopa and entacapone tablets.
11 DESCRIPTION
Carbidopa, levodopa and entacapone tablets is a combination of carbidopa, levodopa, and entacapone for the treatment of Parkinson’s disease.
Carbidopa, an inhibitor of aromatic amino acid decarboxylation, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 244.3. It is designated chemically as (-)-L-(α-hydrazino-(α-methyl-β-(3,4-dihydroxybenzene) propanoic acid monohydrate. Its empirical formula is C10H14N2O4•H2O, and its structural formula is:

Tablet content is expressed in terms of anhydrous carbidopa, which has a molecular weight of 226.3.
Levodopa, an aromatic amino acid, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 197.2. It is designated chemically as (-)-L-α-amino-β-(3,4-dihydroxybenzene) propanoic acid. Its empirical formula is C9H11NO4, and its structural formula is:

Entacapone, a COMT inhibitor, is a nitro-catechol-structured compound with a molecular weight of 305.3. The chemical name of entacapone is (E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide. Its empirical formula is C14H15N3O5 and its structural formula is:

Carbidopa, levodopa and entacapone tablets are supplied as tablets in 6 strengths:
Carbidopa, levodopa and entacapone tablets: 12.5 mg of carbidopa, 50 mg of levodopa and 200 mg of entacapone
Carbidopa, levodopa and entacapone tablets: 18.75 mg of carbidopa, 75 mg of levodopa and 200 mg of entacapone
Carbidopa, levodopa and entacapone tablets: 25 mg of carbidopa, 100 mg of levodopa and 200 mg of entacapone
Carbidopa, levodopa and entacapone tablets: 31.25 mg of carbidopa, 125 mg of levodopa and 200 mg of entacapone
Carbidopa, levodopa and entacapone tablets: 37.5 mg of carbidopa, 150 mg of levodopa and 200 mg of entacapone
Carbidopa, levodopa and entacapone tablets: 50 mg of carbidopa, 200 mg of levodopa and 200 mg of entacapone
Inactive Ingredients: corn starch, microcrystalline cellulose, croscarmellose sodium, glycerol 85%, hypromellose, magnesium stearate, mannitol, polysorbate 80, povidone, sucrose, red iron oxide, and titanium dioxide. Tablets containing 12.5 mg of carbidopa, 50 mg of levodopa and 200 mg of entacapone, tablets containing 25 mg of carbidopa, 100 mg of levodopa and 200 mg of entacapone and tablets containing 37.5 mg of carbidopa, 150 mg of levodopa and 200 mg of entacapone also contain yellow iron oxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Levodopa
Current evidence indicates that symptoms of Parkinson’s disease are related to depletion of dopamine in the corpus striatum. Administration of dopamine is ineffective in the treatment of Parkinson’s disease because it does not cross the blood-brain barrier. However, levodopa the metabolic precursor of dopamine, does cross the blood-brain barrier, and is presumably converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa relieves the symptoms of Parkinson’s disease.
Carbidopa
When levodopa is administered orally, it is rapidly decarboxylated to dopamine in extracerebral tissues so that only a small portion of a given dose is transported unchanged to the central nervous system. Carbidopa inhibits the decarboxylation of peripheral levodopa, making more levodopa available for delivery to the brain.
Entacapone
Entacapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT).
COMT catalyzes the transfer of the methyl group of S-adenosyl-L-methionine to the phenolic group of substrates that contain a catechol structure. Physiological substrates of COMT include DOPA, catecholamines (dopamine, norepinephrine, and epinephrine) and their hydroxylated metabolites. When decarboxylation of levodopa is prevented by carbidopa, COMT becomes the major metabolizing enzyme for levodopa, catalyzing its metabolism to 3-methoxy-4-hydroxy-L-phenylalanine (3-OMD).
12.3 Pharmacokinetics
The pharmacokinetics of carbidopa, levodopa and entacapone tablets has been studied in healthy subjects (age 45 years to 75 years). Overall, following administration of corresponding doses of levodopa, carbidopa and entacapone as carbidopa, levodopa and entacapone tablets or as carbidopa and levodopa product plus Comtan (entacapone) tablets, the mean plasma concentrations of levodopa, carbidopa, and entacapone are comparable.
Absorption and Distribution
Both levodopa and entacapone are rapidly absorbed and eliminated, and their distribution volume is moderately small. Carbidopa is absorbed and eliminated slightly more slowly compared with levodopa and entacapone. There are substantial inter- and intra-individual variations in the absorption of levodopa, carbidopa and entacapone, particularly concerning its Cmax.
The food-effect on the carbidopa, levodopa and entacapone tablet has not been evaluated. Because levodopa competes with certain amino acids for transport across the gut wall, the absorption of levodopa may be impaired in some patients after eating a high protein meal. Meals rich in large neutral amino acids may delay and reduce the absorption of levodopa [see Patient Counseling Information (17)].
Levodopa
The pharmacokinetic properties of levodopa following the administration of single-dose carbidopa, levodopa and entacapone tablets are summarized in Table 3.
| Tablet Strength
| AUC0-∞
(nanogram·h per mL) | Cmax
(nanogram per mL) | Tmax
(h) |
| 12.5 mg per 50 mg per 200 mg | 1,040 ± 314 | 470 ± 154 | 1.1 ± 0.5 |
| 25 mg per 100 mg per 200 mg | 2,910 ± 715 | 975 ± 247 | 1.4 ± 0.6 |
| 37.5 mg per 150 mg per 200 mg | 3,770 ± 1,120 | 1,270 ± 329 | 1.5 ± 0.9 |
| 50 mg per 200 mg per 200 mg | 6,115 ± 1,536 | 1,859 ± 455 | 1.76 ± 0.7 |
Levodopa is bound to plasma protein only to a minor extent (about 10% to 30%).
Carbidopa
Following administration of carbidopa, levodopa and entacapone tablets as a single dose to healthy male and female subjects, the peak concentration of carbidopa was reached within 2.5 hours to 3.4 hours on average. The mean Cmax ranged from about 40 nanogram per mL to 225 nanogram per mL and the mean AUC from 170 nanogram•h per mL to 1,200 nanogram•h per mL, with different carbidopa, levodopa and entacapone tablets strengths providing 12.5 mg, 25 mg, 37.5 mg, or 50 mg of carbidopa.
Carbidopa is approximately 36% bound to plasma protein.
Entacapone
Following administration of carbidopa, levodopa and entacapone tablets as a single dose to healthy male and female subjects, the peak concentration of entacapone in plasma was reached within 0.8 hour to 1.2 hours on average. The mean Cmax of entacapone was about 1,200 nanogram per mL to 1,500 nanogram per mL and the AUC 1,250 nanogram•h per mL to 1,750 nanogram•h per mL after administration of different carbidopa, levodopa and entacapone tablets strengths all providing 200 mg of entacapone.
The plasma protein binding of entacapone is 98% over the concentration range of 0.4 mcg per mL to 50 mcg per mL. Entacapone binds mainly to serum albumin.
Metabolism and Elimination
Levodopa
The elimination half-life of levodopa, the active moiety of antiparkinsonian activity, was 1.7 hours (range 1.1 hours to 3.2 hours).
Levodopa is extensively metabolized to various metabolites. Two major pathways are decarboxylation by dopa decarboxylase (DDC) and O-methylation by COMT.
Carbidopa
The elimination half-life of carbidopa was on average 1.6 hours to 2 hours (range 0.7 hour to 4.0 hours).
Carbidopa is metabolized to two main metabolites (α-methyl-3-methoxy-4-hydroxyphenylpropionic acid and α-methyl-3,4-dihydroxyphenylpropionic acid). These 2 metabolites are primarily eliminated in the urine unchanged or as glucuronide conjugates. Unchanged carbidopa accounts for 30% of the total urinary excretion.
Entacapone
The elimination half-life of entacapone was on average 0.8 hour to 1 hour (0.3 hour to 4.5 hours).
Entacapone is almost completely metabolized prior to excretion with only a very small amount (0.2% of dose) found unchanged in urine. The main metabolic pathway is isomerization to the cis-isomer, the only active metabolite. Entacapone and the cis-isomer are eliminated in the urine as glucuronide conjugates. The glucuronides account for 95% of all urinary metabolites (70% as parent and 25% as cis-isomer glucuronides). The glucuronide conjugate of the cis-isomer is inactive. After oral administration of a 14C-labeled dose of entacapone, 10% of labeled parent and metabolite is excreted in urine and 90% in feces.
Due to short elimination half-lives, no true accumulation of levodopa or entacapone occurs when they are administered repeatedly.
Renal Impairment
Entacapone
The pharmacokinetics of entacapone have been investigated after a single 200 mg entacapone dose in subjects with normal, moderate, and severely impaired renal functions, without levodopa and dopa decarboxylase inhibitor coadministration. No significant effects of renal function on the pharmacokinetics of entacapone were found.
Levodopa and carbidopa
No studies on the pharmacokinetics of levodopa and carbidopa in patients with renal impairment.
Hepatic Impairment
Entacapone
Hepatic impairment had a significant effect on the pharmacokinetics of entacapone when 200 mg entacapone was administered alone. A single 200 mg dose of entacapone, without levodopa and dopa decarboxylase inhibitor coadministration, showed approximately 2-fold higher AUC and Cmax values in patients with a history of alcoholism and hepatic impairment (n=10) compared to normal subjects (n=10). All patients had biopsy-proven liver cirrhosis caused by alcohol. According to Child-Pugh grading 7 patients with liver disease had mild hepatic impairment and 3 patients had moderate hepatic impairment. As only about 10% of the entacapone dose is excreted in urine, as parent compound and conjugated glucuronide, biliary excretion appears to be the major route of excretion of this drug. Carbidopa, levodopa and entacapone tablets should be administered with care to patients with biliary obstruction or hepatic disease.
Levodopa and carbidopa
There are no studies on the pharmacokinetics of levodopa and carbidopa in patients with hepatic impairment.
Geriatric Use
In the pharmacokinetics studies conducted in healthy volunteers following a single dose of carbidopa-, levodopa- and entacapone (as carbidopa, levodopa and entacapone tablets or as separate carbidopa/levodopa and Comtan tablets):
Levodopa
The AUC of levodopa is significantly (on average 10% to 20%) higher in elderly (60 years to 75 years) than younger subjects (45 years to 60 years). There is no significant difference in the Cmax of levodopa between younger (45 years to 60 years) and elderly subjects (60 years to 75 years).
Carbidopa
There is no significant difference in the Cmax and AUC of carbidopa, between younger (45 years to 60 years) and elderly subjects (60 years to 75 years).
Entacapone
The AUC of entacapone is significantly (on average, 15%) higher in elderly (60 years to 75 years) than younger subjects (45 years to 60 years). There is no significant difference in the Cmax of entacapone between younger (45 years to 60 years) and elderly subjects (60 years to 75 years).
Gender
Pharmacokinetics following a single dose of carbidopa, levodopa and entacapone together, either as carbidopa, levodopa and entacapone tablets or as separate carbidopa/levodopa and Comtan tablets in healthy volunteers (age range 45 years to 74 years):
Levodopa
The plasma exposure (AUC and Cmax) of levodopa is significantly higher in females than males (on average, 40% for AUC and 30% for Cmax). These differences are primarily explained by body weight. Other published literature showed significant gender effect (higher concentrations in females) even after correction for body weight.
Carbidopa
There is no gender difference in the pharmacokinetics of carbidopa.
Entacapone
There is no gender difference in the pharmacokinetics of entacapone.
Drug Interaction Studies
Drug Metabolized by COMT
When a single 400 mg dose of entacapone was given together with intravenous isoprenaline (isoproterenol) and epinephrine without coadministered levodopa and dopa decarboxylase inhibitor, the overall mean maximal changes in heart rate during infusion were about 50% and 80% higher than with placebo, for isoprenaline and epinephrine, respectively.
Drugs known to be metabolized by COMT should be administered with caution in patients receiving entacapone regardless of the route of administration [see Drug Interactions (7.2)].
Drugs Metabolized via CYP2C9
Due to its affinity to CYP2C9 in vitro, entacapone may potentially interfere with medicinal products with metabolism dependent on this isoenzyme. In an interaction study in healthy volunteers, entacapone increased the AUC of R-warfarin on average by 18%, and the INR values increased on average by 13% [see Drug Interactions (7.11)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In rats, oral administration of carbidopa-levodopa for 2 years resulted in no evidence of carcinogenicity at doses of approximately 2 times (carbidopa)-4 times (levodopa) the maximum recommended human dose (MRHD).
Two-year carcinogenicity studies of entacapone were conducted in mice and rats. In mice, no increase in tumors was observed at oral doses of 100, 200 and 400 mg/kg/day. At the highest dose tested, plasma exposures (AUC) were 4 times higher than that in humans at the maximum recommended daily dose (MRDD) of 1,600 mg. In rats administered oral doses of 20, 90, or 400 mg/kg/day, an increased incidence of renal tubular adenomas and carcinomas was observed in males at the highest dose tested. Plasma AUCs at the higher dose not associated with increased renal tumors (90 mg/kg/day) were approximately 5 times that in humans at the MRDD of entacapone.
The carcinogenic potential of entacapone administered in combination with carbidopa-levodopa has not been evaluated.
Mutagenesis
Carbidopa was mutagenic in the in vitro bacterial reverse mutation (Ames) assay in the presence and absence of metabolic activation, and in the in vitro mouse lymphoma thymidine kinase (tk) assay in the absence of metabolic activation. Carbidopa was negative in the in vivo mouse micronucleus assay.
Entacapone was mutagenic and clastogenic in the in vitro mouse lymphoma tk assay in the presence and absence of metabolic activation, and was clastogenic in cultured human lymphocytes in the presence of metabolic activation. Entacapone, either alone or in combination with carbidopa-levodopa, was negative in the in vivo mouse micronucleus and in the Ames assays.
Impairment of Fertility
In reproduction studies, no effects on fertility were found in rats receiving carbidopa-levodopa at doses of approximately 2 times (carbidopa)-4 times (levodopa) the MRHD.
In rats treated orally with entacapone (up to 700 mg/kg/day), no effects on fertility or general reproductive performance were observed. Plasma exposures (AUC) at the highest dose tested were approximately 30 times that in humans at the MRHD of entacapone. Delayed mating was evident in females at the highest dose tested.
14 CLINICAL STUDIES
The effectiveness of entacapone as an adjunct to levodopa in the treatment of Parkinson’s disease was established in three 24-week multicenter, randomized, double-blind, placebo-controlled studies in patients with Parkinson’s disease. In 2 of these studies (Studies 1 and 2), the patients’ disease was “fluctuating”, i.e., was characterized by documented periods of “On” (periods of relatively good functioning) and “Off” (periods of relatively poor functioning), despite optimum levodopa therapy. There was also a withdrawal period following 6 months of treatment. In the third trial patients were not required to have been experiencing fluctuations. Prior to the controlled part of these studies, patients were stabilized on levodopa for 2 weeks to 4 weeks.
There is limited experience using entacapone in patients who do not experience fluctuations.
In Studies 1 and 2, patients were randomized to receive placebo or entacapone 200 mg administered concomitantly with each dose of carbidopa/levodopa (up to 10 times daily, but patients averaged 4 doses to 6 doses per day). The double-blind portion of both studies was 6 months long. Patients periodically recorded the time spent in the “On” and “Off” states in home diaries throughout the duration of the trial. In one study, conducted in the Nordic countries, the primary outcome measure was the total mean time spent in the “On” state during an 18 hour diary recorded day (6 a.m. to midnight). In the other study, the primary outcome measure was the proportion of awake time spent over 24 hours in the “On” state.
In addition to the primary outcome measure, the amount of time spent in the “Off” state was evaluated, and patients were also evaluated by subparts of the Unified Parkinson’s Disease Rating Scale (UPDRS), a frequently used multi-item rating scale intended to assess mentation (Part I), activities of daily living (Part II), motor function (Part III), complications of therapy (Part IV), and disease staging (Part V and VI); an investigator’s and patient’s global assessment of clinical condition, a 7-point subjective scale designed to assess global functioning in Parkinson’s disease; and the change in daily carbidopa/levodopa dose.
In Study 1, 171 patients were randomized in 16 centers in Finland, Norway, Sweden, and Denmark (Study 1), all of whom received concomitant levodopa plus dopa decarboxylase inhibitor (either carbidopa/levodopa or benserazide/levodopa). In Study 2, 205 patients were randomized in 17 centers in North America (US and Canada); all patients received concomitant carbidopa/levodopa.
The following tables (Table 4 and Table 5) display the results of these two studies:
| *Mean; the month 6 values represent the average of weeks 8, 16, and 24, by protocol-defined outcome measure. **At least one category change at endpoint. ***Not an endpoint for this study but primary endpoint in the North American Study. ‡Not significant. ‡‡P values for Secondary Measures are nominal P values without any adjustment for multiplicity. |
|||
| Primary Measure from Home Diary (from an 18-hour Diary Day)
|
|||
| | Baseline | Change from Baseline at Month 6* | p-value vs. placebo |
| Hours of Awake Time “On”
|
|||
| Placebo |
9.2 | +0.1 | – |
| Entacapone | 9.3 | +1.5 | less than 0.001 |
| Duration of “On” Time After First AM Dose (Hrs)
|
|||
| Placebo | 2.2 | 0.0 | - |
| Entacapone | 2.1 | +0.2 | less than 0.05 |
| Secondary Measures from Home Diary (from an 18-hour Diary Day)‡‡ |
|||
| Hours of Awake Time “Off”
|
|||
| Placebo | 5.3 | 0.0 | – |
| Entacapone | 5.5 | -1.3 | less than 0.001 |
| Proportion of Awake Time “On”*** (%)
|
|||
| Placebo
| 63.8 | +0.6 | – |
| Entacapone | 62.7 | +9.3 | less than 0.001 |
| Levodopa Total Daily Dose (mg)
|
|||
| Placebo
| 705 | +14 | – |
| Entacapone | 701 | -87 | less than 0.001 |
| Frequency of Levodopa Daily Intakes
|
|||
| Placebo
| 6.1 | +0.1 | – |
| Entacapone | 6.2 | -0.4 | less than 0.001 |
| Other Secondary Measures‡‡
|
|||
| Baseline | Change from Baseline at Month 6 | p-value vs. placebo |
|
| Investigator’s Global (overall) % Improved**
|
|||
| Placebo | – | 28 | - |
| Entacapone | – | 56 | less than 0.01 |
| Patient’s Global (overall) % Improved** |
|||
| Placebo | - | 22 | - |
| Entacapone | - | 39 | N.S.‡ |
| UPDRS Total
|
|||
| Placebo | 37.4 | -1.1 | - |
| Entacapone | 38.5 | -4.8 | less than 0.01 |
| UPDRS Motor
|
|||
| Placebo
| 24.6 | -0.7 | – |
| Entacapone | 25.5 | -3.3 | less than 0.05 |
| UPDRS ADL
|
|||
| Placebo
| 11.0 | -0.4 | – |
| Entacapone | 11.2 | -1.8 | less than 0.05 |
| *Mean; the month 6 values represent the average of weeks 8, 16, and 24, by protocol-defined outcome measure. **At least one category change at endpoint. ***Score change at endpoint similarly to the Nordic Study. ‡Not significant. ‡‡P values for Secondary Measures are nominal P values without any adjustment for multiplicity. |
|||
| Primary Measure from Home Diary (for a 24-hour Diary Day)
|
|||
| | Baseline | Change from Baseline at Month 6* | p-value vs. placebo |
| Percent of Awake Time “On”
|
|||
| Placebo | 60.8 | +2.0 | - |
| Entacapone | 60.0 | +6.7 | less than 0.05 |
| Secondary Measures from Home Diary (for a 24-hour Diary Day)‡‡
|
|||
| Hours of Awake Time “Off”
|
|||
| Placebo | 6.6 | -0.3 | – |
| Entacapone | 6.8 | -1.2 | less than 0.01 |
| Hours of Awake Time “On”
|
|||
| Placebo | 10.3 | +0.4 | – |
| Entacapone | 10.2 | +1.0 | N.S.‡ |
| Levodopa Total Daily Dose (mg)
Placebo Entacapone | 758 804 | +19 -93 | - less than 0.001 |
| Frequency of Levodopa Daily Intakes
| | | |
| Placebo | 6.0 | +0.2 | – |
| Entacapone | 6.2 | 0.0 | N.S.‡ |
| Other Secondary Measures‡‡
|
|||
| | Baseline | Change from Baseline at Month 6 | p-value vs. placebo |
| Investigator’s Global (overall) % Improved**
| | | |
| Placebo | - | 21 | - |
| Entacapone | - | 34 | less than 0.05 |
| Patient’s Global (overall) % Improved**
| | | |
| Placebo | - | 20 | – |
| Entacapone | - | 31 | less than 0.05 |
| UPDRS Total***
| | | |
| Placebo | 35.6 | +2.8 | – |
| Entacapone | 35.1 | -0.6 | less than 0.05 |
| UPDRS Motor***
| | | |
| Placebo | 22.6 | +1.2 | – |
| Entacapone | 22.0 | -0.9 | less than 0.05 |
| UPDRS ADL***
| | | |
| Placebo | 11.7 | +1.1 | – |
| Entacapone | 11.9 | 0.0 | less than 0.05 |
Effects on “On” time did not differ by age, sex, weight, disease severity at baseline, levodopa dose and concurrent treatment with dopamine agonists or selegiline.
Withdrawal of entacapone:
In Study 2, abrupt withdrawal of entacapone, without alteration of the dose of carbidopa/levodopa, resulted in a significant worsening of fluctuations, compared to placebo. In some cases, symptoms were slightly worse than at baseline, but returned to approximately baseline severity within 2 weeks following levodopa dose increase on average by 80 mg. In Study 1, similarly, a significant worsening of parkinsonian symptoms was observed after entacapone withdrawal, as assessed 2 weeks after drug withdrawal. At this phase, the symptoms were approximately at baseline severity following levodopa dose increase by about 50 mg.
In the third placebo-controlled trial (Study 3), a total of 301 patients were randomized in 32 centers in Germany and Austria. In this trial, as in the other 2 studies, entacapone 200 mg was administered with each dose of levodopa and dopa decarboxylase inhibitor (up to 10 times daily) and UPDRS Parts II and III and total daily “On” time were the primary measures of effectiveness. Results for the primary measures, as well as for some secondary measures are presented in Table 6.
| Primary Measures
|
|||
| | Baseline | Change from Baseline at Month 6 | p-value vs. placebo (LOCF) |
| UPDRS ADL*
|
|||
| Placebo | 12.0 | +0.5 | – |
| Entacapone | 12.4 | -0.4 | less than 0.05 |
| UPDRS Motor*
|
|||
| Placebo | 24.1 | +0.1 | – |
| Entacapone | 24.9 | -2.5 | less than 0.05 |
| Hours of Awake Time “On” (Home Diary)**
|
|||
| Placebo | 10.1 | +0.5 | – |
| Entacapone | 10.2 | +1.1 | N.S.‡ |
| Secondary Measures‡‡
|
|||
| | Baseline | Change from Baseline at Month 6 | p-value vs. placebo |
| UPDRS Total*
|
|||
| Placebo | 37.7 | +0.6 | – |
| Entacapone | 39.0 | -3.4 | less than 0.05 |
| Percent of Awake Time “On” (Home Diary)**
|
|||
| Placebo | 59.8 | +3.5 | – |
| Entacapone | 62.0 | +6.5 | N.S.‡ |
| Hours of Awake Time “Off” (Home Diary)**
|
|||
| Placebo | 6.8 | -0.6 | – |
| Entacapone | 6.3 | -1.2 | 0.07 |
| Levodopa Total Daily Dose (mg)*
|
|||
| Placebo | 572 | +4 | – |
| Entacapone | 566 | -35 | N.S.‡ |
| Frequency of Levodopa Daily Intake*
|
|||
| Placebo | 5.6 | +0.2 | – |
| Entacapone | 5.4 | 0.0 | less than 0.01 |
| Global (overall) % Improved***
|
|||
| Placebo | – | 34 | – |
| Entacapone | – | 38 | N.S.‡ |
| *Total population; score change at endpoint. **Fluctuating population, with 5 doses to 10 doses; score change at endpoint. ***Total population; at least one category change at endpoint. ‡Not significant. ‡‡P values for Secondary Measures are nominal P values without any adjustment for multiplicity. |
|||
16 HOW SUPPLIED/STORAGE AND HANDLING
Carbidopa, levodopa and entacapone tablets are supplied as film-coated tablets for oral administration in the following six strengths:
Carbidopa, levodopa and entacapone film-coated tablets 12.5 mg/50 mg/200 mg containing 12.5 mg of carbidopa, 50 mg of levodopa and 200 mg of entacapone.
The unscored, round, biconvex shaped tablets are brownish and debossed “S 50” on one side and plain on other side.
HDPE bottle of 100 tablets NDC 16571-689-01.
Carbidopa, levodopa and entacapone film-coated tablets 18.75 mg/75 mg/200 mg containing 18.75 mg of carbidopa, 75 mg of levodopa and 200 mg of entacapone.
The unscored, oval, biconvex shaped tablets are light brownish and debossed “S75” on one side and plain on other side.
HDPE bottle of 100 tablets NDC 16571-690-01.
Carbidopa, levodopa and entacapone film-coated tablets 25 mg/100 mg/200 mg containing 25 mg of carbidopa, 100 mg of levodopa and 200 mg of entacapone.
The unscored, oval, biconvex shaped tablets are brownish and debossed “S100” on one side and plain on other side.
HDPE bottle of 100 tablets NDC 16571-691-01.
Carbidopa, levodopa and entacapone film-coated tablets 31.25 mg/125 mg/200 mg containing 31.25 mg of carbidopa, 125 mg of levodopa and 200 mg of entacapone.
The unscored, oval, biconvex shaped tablets are light brownish and debossed “S125” on one side and plain on other side.
HDPE bottle of 100 tablets NDC 16571-692-01.
Carbidopa, levodopa and entacapone film-coated tablets 37.5 mg/150 mg/200 mg containing 37.5 mg of carbidopa, 150 mg of levodopa and 200 mg of entacapone.
The unscored, elongated ellipse shaped tablets are brownish and debossed “S150” on one side and plain on other side.
HDPE bottle of 100 tablets NDC 16571-693-01.
Carbidopa, levodopa and entacapone film-coated tablets 50 mg/200 mg/200 mgcontaining 50 mg of carbidopa, 200 mg of levodopa and 200 mg of entacapone.
The unscored, oval shaped tablets are brownish red and debossed “S200” on one side and plain on other side.
HDPE bottle of 100 tablets NDC 16571-694-01.
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
[See USP Controlled Room Temperature.]
Dispense in tight container (USP).
17 PATIENT COUNSELING INFORMATION
Falling Asleep During Activities of Daily Living and Somnolence
Advise patients about the potential for sedating effects associated with carbidopa, levodopa and entacapone tablets including somnolence and the possibility of falling asleep while engaged in activities of daily living. Because somnolence can be a frequent adverse reaction with potentially serious consequences, patients should not drive a motor vehicle, operate heavy machinery or engage in other potentially dangerous activities until they have gained sufficient experience with carbidopa, levodopa and entacapone tablets to determine whether or not it affects their mental and/or motor performance adversely [see Warnings and Precautions (5.1)]. Advise patients that if increased somnolence or episodes of falling asleep during activities of daily living (e.g., conversations, eating, driving a motor vehicle, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician.
Advise patients to speak with their health care prescriber before taking alcohol, sedating medications, or other CNS depressants (e.g., benzodiazepines, antipsychotics, antidepressants, etc.) because of the possible additive effects in combination with carbidopa, levodopa and entacapone tablets.
Hypotension, Orthostatic Hypotension and Syncope
Advise patients that they may develop symptomatic (or asymptomatic) postural (orthostatic) hypotension or non-orthostatic hypotension while taking carbidopa, levodopa and entacapone tablets. Hypotension/orthostatic hypotension may occur more frequently during initial therapy. Patients should not rise rapidly after sitting or lying down, especially if they have been doing so for prolonged periods and especially at the initiation of treatment with carbidopa, levodopa and entacapone tablets.
Advise patients about the potential for syncope in patients using dopamine agonists. For this reason, inform patients about the possibility of syncope while taking carbidopa, levodopa and entacapone tablets [see Warnings and Precautions (5.2)].
Dyskinesias
Inform patients that carbidopa, levodopa and entacapone tablets may cause and/or exacerbate pre-existing dyskinesias [see Warnings and Precautions (5.3)].
Hallucinations and/or Psychotic-Like Behavior
Inform patients that hallucinations and other psychotic-like behavior can occur while taking carbidopa, levodopa and entacapone tablets [see Warnings and Precautions (5.5)].
Impulse Control and/or Compulsive Behaviors
Advise patients that they may experience impulse control and/or compulsive behaviors while taking one or more of the medications used for the treatment of Parkinson’s disease, including carbidopa, levodopa and entacapone tablets. Ask patients about the development of new or increased gambling urges, sexual urges, urges for uncontrolled spending, or other intense urges while being treated with carbidopa, levodopa and entacapone tablets. Advise patients to inform their physician or health care provider if they experience new or increased gambling urges, increased sexual urges or other intense urges while taking carbidopa, levodopa and entacapone tablets. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking carbidopa, levodopa and entacapone tablets [see Warnings and Precautions (5.6)].
Withdrawal-Emergent Hyperpyrexia and Confusion
Advise patients that they may develop fever and confusion as part of a syndrome resembling NMS and possibly with other clinical features (e.g., muscle rigidity, autonomic dysfunction, hyper- or hypotension, etc.). This fever and confusion syndrome may particularly occur with dose reductions or withdrawal of carbidopa, levodopa and entacapone tablets but may also develop after initiation of treatment. Advise patients to contact their healthcare provider if they wish to discontinue or decrease the dose of carbidopa, levodopa and entacapone tablets and to contact a health care provider if they develop fever and confusion [see Warnings and Precautions (5.7)].
Diarrhea and Colitis
Inform the patients that diarrhea may occur with carbidopa, levodopa and entacapone tablets and it may have a delayed onset. Sometimes prolonged diarrhea may be caused by colitis (inflammation of the large intestine). Patients with diarrhea should drink fluids to maintain adequate hydration and monitor for weight loss. If diarrhea associated with carbidopa, levodopa and entacapone tablets is prolonged, discontinuing the drug is expected to lead to resolution. If diarrhea continues after stopping carbidopa, levodopa and entacapone tablets, further diagnostic investigations may be needed. In some cases, diarrhea may be associated with colitis [see Warnings and Precautions (5.8)].
Rhabdomyolysis
Advise patients that they may develop rhabdomyolysis and myalgia if they experience prolonged motor activity including dyskinesia. This event may also be associated with fever and confusion [see Warnings and Precautions (5.9)].
Nausea and Vomiting
Inform patients that carbidopa, levodopa and entacapone tablets may cause nausea and vomiting may occur more frequently during initial therapy and may require dose adjustment.
Instructions for Use
Instruct patients to take carbidopa, levodopa and entacapone tablets only as prescribed. Instruct patients to only take a single tablet of carbidopa, levodopa and entacapone tablets at each dosing interval. Instruct patients not to take multiple tablets or additional portions of tablets to achieve a higher dose of levodopa. Advise patients not to split, crush, or chew carbidopa, levodopa and entacapone tablets.
Inform the patient that carbidopa, levodopa and entacapone tablets is a formulation of carbidopa/levodopa combined with entacapone that is designed to begin release of ingredients within 30 minutes after ingestion. It is important that carbidopa, levodopa and entacapone tablets be taken at regular intervals according to the schedule outlined by the physician. Caution the patient not to change the prescribed dosage regimen and not to add any additional antiparkinsonian medications, including other carbidopa/levodopa preparations, without first consulting the physician.
Advise patients that “off” episodes (“wearing-off” of drug effect) occur at the end of the dosing interval but unpredictable “off” episodes may occur at any time. Advise the patient to notify a health care provider for possible treatment adjustments if such response poses a problem to the patient’s everyday life.
Advise patients that dark coloration (red, brown, or black) may appear in saliva, urine, or sweat after taking carbidopa, levodopa and entacapone tablets. Although the color appears to be clinically insignificant, garments may become discolored.
Advise patients that a change in diet to foods that are high in protein may delay the absorption of levodopa. Excessive acidity also delays stomach emptying, thus delaying the absorption of levodopa. Iron salts (such as in multi-vitamin tablets) may also reduce the effectiveness of carbidopa, levodopa and entacapone tablets.
Pregnancy
Instruct patients to notify their healthcare provider if they become pregnant or intend to become pregnant during therapy [see Use in Specific Populations (8.1)].
Lactation
Instruct patients to notify their healthcare provider if they intend to breastfeed or are breastfeeding an infant [see Use in Specific Populations (8.2)].
Manufactured for:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Made in India
Manufactured for:
Suven Pharmaceuticals Limited,
Pashamylaram, Telangana
502307, India.
M.L No.: 24/MD/AP/2009/F/CC
Revised: 09/2021
PIR69401-03
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - (100 Tablets Bottle)
Rising® NDC 16571-689-01
Carbidopa, Levodopa and Entacapone Tablets
Carbidopa, USP 12.5 mg
Levodopa, USP 50 mg
and
Entacapone, USP 200 mg
100 Tablets Rx Only
Do not combine tablets to achieve a higher strength tablet due to the risk of entacapone overdose.

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - (100 Tablets Bottle)
Rising® NDC 16571-690-01
Carbidopa, Levodopa and Entacapone Tablets
Carbidopa, USP 18.75 mg
Levodopa, USP 75 mg
and
Entacapone, USP 200 mg
100 Tablets Rx Only
Do not combine tablets to achieve a higher strength tablet due to the risk of entacapone overdose.

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - (100 Tablets Bottle)
Rising® NDC 16571-691-01
Carbidopa, Levodopa and Entacapone Tablets
Carbidopa, USP 25 mg
Levodopa, USP 100 mg
and
Entacapone, USP 200 mg
100 Tablets Rx Only
Do not combine tablets to achieve a higher strength tablet due to the risk of entacapone overdose.

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - (100 Tablets Bottle)
Rising® NDC 16571-692-01
Carbidopa, Levodopa and Entacapone Tablets
Carbidopa, USP 31.25 mg
Levodopa, USP 125 mg
and
Entacapone, USP 200 mg
100 Tablets Rx Only
Do not combine tablets to achieve a higher strength tablet due to the risk of entacapone overdose.

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - (100 Tablets Bottle)
Rising® NDC 16571-693-01
Carbidopa, Levodopa and Entacapone Tablets
Carbidopa, USP 37.5 mg
Levodopa, USP 150 mg
and
Entacapone, USP 200 mg
100 Tablets Rx Only
Do not combine tablets to achieve a higher strength tablet due to the risk of entacapone overdose.

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - (100 Tablets Bottle)
Rising® NDC 16571-694-01
Carbidopa, Levodopa and Entacapone Tablets
Carbidopa, USP 50 mg
Levodopa, USP 200 mg
and
Entacapone, USP 200 mg
100 Tablets Rx Only
Do not combine tablets to achieve a higher strength tablet due to the risk of entacapone overdose.

INGREDIENTS AND APPEARANCE
| CARBIDOPA, LEVODOPA AND ENTACAPONE
carbidopa, levodopa and entacapone tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CARBIDOPA, LEVODOPA AND ENTACAPONE
carbidopa, levodopa and entacapone tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| CARBIDOPA, LEVODOPA AND ENTACAPONE
carbidopa, levodopa and entacapone tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CARBIDOPA, LEVODOPA AND ENTACAPONE
carbidopa, levodopa and entacapone tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| CARBIDOPA, LEVODOPA AND ENTACAPONE
carbidopa, levodopa and entacapone tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CARBIDOPA, LEVODOPA AND ENTACAPONE
carbidopa, levodopa and entacapone tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Rising Pharma Holdings, Inc. (835513529) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Suven Life Sciences | 677604288 | ANALYSIS(16571-689, 16571-690, 16571-691, 16571-692, 16571-693, 16571-694) , LABEL(16571-689, 16571-690, 16571-691, 16571-692, 16571-693, 16571-694) , MANUFACTURE(16571-689, 16571-690, 16571-691, 16571-692, 16571-693, 16571-694) , PACK(16571-689, 16571-690, 16571-691, 16571-692, 16571-693, 16571-694) | |