Search by Drug Name or NDC
NDC 16714-0467-01 CAPECITABINE 150 mg/1 Details
CAPECITABINE 150 mg/1
CAPECITABINE is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by NORTHSTAR RXLLC. The primary component is CAPECITABINE.
MedlinePlus Drug Summary
Capecitabine is used in combination with other medications to treat breast cancer that has come back after treatment with other medications. It is also used alone to treat breast cancer that has not improved after treatment with other medications. Capecitabine is also used to treat colon or rectal cancer (cancer that begins in the large intestine) that has gotten worse or spread to other parts of the body. It is also used to prevent colon cancer from spreading in people who have had surgery to remove the tumor. Capecitabine is in a class of medications called antimetabolites. It works by stopping or slowing the growth of cancer cells.
Related Packages: 16714-0467-01Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Capecitabine
Product Information
| NDC | 16714-0467 |
|---|---|
| Product ID | 16714-467_d6937464-feb2-ada0-e053-2a95a90a3aef |
| Associated GPIs | 21300005000320 |
| GCN Sequence Number | 039780 |
| GCN Sequence Number Description | capecitabine TABLET 150 MG ORAL |
| HIC3 | V1B |
| HIC3 Description | ANTINEOPLASTIC - ANTIMETABOLITES |
| GCN | 31611 |
| HICL Sequence Number | 018385 |
| HICL Sequence Number Description | CAPECITABINE |
| Brand/Generic | Generic |
| Proprietary Name | CAPECITABINE |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | CAPECITABINE |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 150 |
| Active Ingredient Units | mg/1 |
| Substance Name | CAPECITABINE |
| Labeler Name | NORTHSTAR RXLLC |
| Pharmaceutical Class | Nucleic Acid Synthesis Inhibitors [MoA], Nucleoside Metabolic Inhibitor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA202593 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 16714-0467-01 (16714046701)
| NDC Package Code | 16714-467-01 |
|---|---|
| Billing NDC | 16714046701 |
| Package | 60 TABLET, FILM COATED in 1 BOTTLE, PLASTIC (16714-467-01) |
| Marketing Start Date | 2015-11-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 7b260da3-dee2-4bbb-9235-39b16720d578 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
CAPECITABINE tablets, for oral use
Initial U.S. Approval: 1998
WARNING: CAPECITABINE -WARFARIN INTERACTION
See full prescribing information for complete boxed warning.
Patients receiving concomitant capecitabine and oral coumarin-derivative anticoagulants such as warfarin and phenprocoumon should have their anticoagulant response (INR or prothrombin time) monitored frequently in order to adjust the anticoagulant dose accordingly. Altered coagulation parameters and/or bleeding, including death, have been reported during concomitant use.
- Occurrence: Within several days and up to several months after initiating capecitabine therapy; may also be seen within 1 month after stopping capecitabine
- Predisposing factors: age>60 and diagnosis of cancer
INDICATIONS AND USAGE
Capecitabine is a nucleoside metabolic inhibitor with antineoplastic activity indicated for:
-
Adjuvant Colon Cancer (
1.1)
- Patients with Dukes' C colon cancer
-
Metastatic Colorectal Cancer (
1.1)
- First-line as monotherapy when treatment with fluoropyrimidine therapy alone is preferred
-
Metastatic Breast Cancer (
1.2)
- In combination with docetaxel after failure of prior anthracycline-containing therapy
- As monotherapy in patients resistant to both paclitaxel and an anthracycline-containing regimen
DOSAGE AND ADMINISTRATION
- Take capecitabine tablets with water within 30 min after a meal ( 2.1)
- Monotherapy: 1250 mg/m 2 twice daily orally for 2 weeks followed by a one week rest period in 3-week cycles ( 2.2)
- Adjuvant treatment is recommended for a total of 6 months (8 cycles) ( 2.2)
- In combination with docetaxel, the recommended dose of capecitabine tablet is 1250 mg/m 2 twice daily for 2 weeks followed by a 7-day rest period, combined with docetaxel at 75 mg/m 2 as a 1-hour IV infusion every 3 weeks ( 2.2)
- Capecitabine tablets dosage may need to be individualized to optimize patient management ( 2.3)
- Reduce the dose of capecitabine tablets by 25% in patients with moderate renal impairment ( 2.4)
DOSAGE FORMS AND STRENGTHS
- Tablets: 150 mg and 500 mg ( 3)
WARNINGS AND PRECAUTIONS
- Coagulopathy:: May result in bleeding, death. Monitor anticoagulant response (e.g., INR) and adjust anticoagulant dose accordingly.( 5.1)
- Diarrhea: May be severe. Interrupt capecitabine treatment immediately until diarrhea resolves or decreases to grade 1. Recommend standard antidiarrheal treatments. ( 5.2)
- Cardiotoxicity: Common in patients with a prior history of coronary artery disease. ( 5.3)
- Increased Risk of Severe or Fatal Adverse Reactions in Patients with Low or Absent Dihydropyrimidine Dehydrogenase (DPD) Activity: Withhold or permanently discontinue capecitabine in patients with evidence of acute early-onset or unusually severe toxicity, which may indicate near complete or total absence of DPD activity. No capecitabine dose has been proven safe in patients with absent DPD activity. ( 5.4)
- Dehydration and Renal Failure: Interrupt capecitabine treatment until dehydration is corrected. Potential risk of acute renal failure secondary to dehydration. Monitor and correct dehydration. ( 5.5)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. ( 5.6, 8.1, 8.3)
- Mucocutaneous and Dermatologic Toxicity: Severe mucocutaneous reactions, Steven-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), have been reported. Capecitabine should be permanently discontinued in patients who experience a severe mucocutaneous reaction during treatment. Capecitabine may induce hand-and-foot syndrome. Persistent or severe hand-and-foot syndrome can lead to loss of fingerprints which could impact patient identification. Interrupt capecitabine treatment until the hand-and-foot syndrome event resolves or decreases in intensity.( 5.7)
- Hyperbilirubinemia: Interrupt capecitabine treatment immediately until the hyperbilirubinemia resolves or decreases in intensity. ( 5.8)
- Hematologic: Do not treat patients with neutrophil counts <1.5 × 10 9/L or thrombocyte counts <100 × 10 9/L. If grade 3 to 4 neutropenia or thrombocytopenia occurs, stop therapy until condition resolves. ( 5.9)
ADVERSE REACTIONS
Most common adverse reactions (≥30%) were diarrhea, hand-and-foot syndrome, nausea, vomiting, abdominal pain, fatigue/weakness, and hyperbilirubinemia. Other adverse reactions, including serious adverse reactions, have been reported. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Northstar RxLLC at 1-800-206-7821 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Anticoagulants: Monitor anticoagulant response (INR or prothrombin time) frequently in order to adjust the anticoagulant dose as needed. ( 5.2, 7.1)
- Phenytoin: Monitor phenytoin levels in patients taking capecitabine concomitantly with phenytoin. The phenytoin dose may need to be reduced. ( 7.1)
- Leucovorin: The concentration of 5-fluorouracil is increased and its toxicity may be enhanced by leucovorin. ( 7.1)
- CYP2C9 substrates: Care should be exercised when capecitabine is coadministered with CYP2C9 substrates. ( 7.1)
- Allopurinol: Avoid the use of allopurinol during treatment with capecitabine.
- Food reduced both the rate and extent of absorption of capecitabine. ( 2, 7.2, 12.3)
USE IN SPECIFIC POPULATIONS
- Lactation: Advise women not to breastfeed.. ( 8.2)
- Females and Males of Reproductive Potential: Verify pregnancy status of females prior to initiation of capecitabine. Advise males with female partners of reproductive potential to use effective contraception. ( 8.3)
- Geriatric: Greater incidence of adverse reactions. Monitoring required. ( 8.5)
- Hepatic Impairment: Monitoring is recommended in patients with mild to moderate hepatic impairment. ( 8.6)
- Renal Impairment: Reduce capecitabine starting dose in patients with moderate renal impairment ( 2.4, 8.7, 12.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CAPECITABINE-WARFARIN INTERACTION
1 INDICATIONS AND USAGE
1.1 Colorectal Cancer
1.2 Breast Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Standard Starting Dose
2.3 Dose Management Guidelines
2.4 Adjustment of Starting Dose in Special Populations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Severe Renal Impairment
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Coagulopathy
5.2 Diarrhea
5.3 Cardiotoxicity
5.4 Dihydropyrimidine Dehydrogenase Deficiency
5.5 Dehydration and Renal Failure
5.6 Embryo-Fetal Toxicity
5.7 Mucocutaneous and Dermatologic Toxicity
5.8 Hyperbilirubinemia
5.9 Hematologic
5.10 Geriatric Patients
5.11 Hepatic Insufficiency
5.12 Combination With Other Drugs
6 ADVERSE REACTIONS
6.1 Adjuvant Colon Cancer
6.2 Metastatic Colorectal Cancer
6.3 Breast Cancer
6.4 Clinically Relevant Adverse Events in <5% of Patients
6.5 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
7.2 Drug-Food Interaction
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Insufficiency
8.7 Renal Insufficiency
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adjuvant Colon Cancer
14.2 Metastatic Colorectal Cancer
14.3 Breast Cancer
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
Capecitabine Warfarin Interaction: Patients receiving concomitant capecitabine and oral coumarin-derivative anticoagulant therapy should have their anticoagulant response (INR or prothrombin time) monitored frequently in order to adjust the anticoagulant dose accordingly. A clinically important Capecitabine-Warfarin drug interaction was demonstrated in a clinical pharmacology trial [see Warnings and Precautions (5.2) and Drug Interactions (7.1)] . Altered coagulation parameters and/or bleeding, including death, have been reported in patients taking capecitabine concomitantly with coumarin-derivative anticoagulants such as warfarin and phenprocoumon. Postmarketing reports have shown clinically significant increases in prothrombin time (PT) and INR in patients who were stabilized on anticoagulants at the time capecitabine was introduced. These events occurred within several days and up to several months after initiating capecitabine therapy and, in a few cases, within 1 month after stopping capecitabine. These events occurred in patients with and without liver metastases. Age greater than 60 and a diagnosis of cancer independently predispose patients to an increased risk of coagulopathy.
1 INDICATIONS AND USAGE
1.1 Colorectal Cancer
- Capecitabine tablets, USP are indicated as a single agent for adjuvant treatment in patients with Dukes' C colon cancer who have undergone complete resection of the primary tumor when treatment with fluoropyrimidine therapy alone is preferred. Capecitabine was non-inferior to 5-fluorouracil and leucovorin (5-FU/LV) for disease-free survival (DFS). Physicians should consider results of combination chemotherapy trials, which have shown improvement in DFS and OS, when prescribing single-agent capecitabine in the adjuvant treatment of Dukes' C colon cancer.
- Capecitabine tablets, USP are indicated as first-line treatment of patients with metastatic colorectal carcinoma when treatment with fluoropyrimidine therapy alone is preferred. Combination chemotherapy has shown a survival benefit compared to 5-FU/LV alone. A survival benefit over 5-FU/LV has not been demonstrated with capecitabine monotherapy. Use of capecitabine tablets, USP instead of 5-FU/LV in combinations has not been adequately studied to assure safety or preservation of the survival advantage.
1.2 Breast Cancer
- Capecitabine tablets, USP in combination with docetaxel are indicated for the treatment of patients with metastatic breast cancer after failure of prior anthracycline-containing chemotherapy.
- Capecitabine tablets, USP monotherapy is also indicated for the treatment of patients with metastatic breast cancer resistant to both paclitaxel and an anthracycline-containing chemotherapy regimen or resistant to paclitaxel and for whom further anthracycline therapy is not indicated (e.g., patients who have received cumulative doses of 400 mg/m 2 of doxorubicin or doxorubicin equivalents). Resistance is defined as progressive disease while on treatment, with or without an initial response, or relapse within 6 months of completing treatment with an anthracycline-containing adjuvant regimen.
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Capecitabine tablets should be swallowed whole with water within 30 minutes after a meal. Capecitabine tablets are cytotoxic drug. Follow applicable special handling and disposal procedures. 1 If capecitabine tablets must be cut or crushed, this should be done by a professional trained in safe handling of cytotoxic drugs using appropriate equipment and safety procedures. Capecitabine tablets dose is calculated according to body surface area.
2.2 Standard Starting Dose
Monotherapy (Metastatic Colorectal Cancer, Adjuvant Colorectal Cancer, Metastatic Breast Cancer)
The recommended dose of capecitabine tablets are 1250 mg/m 2 administered orally twice daily (morning and evening; equivalent to 2500 mg/m 2 total daily dose) for 2 weeks followed by a 1-week rest period given as 3-week cycles (see Table 1).
Adjuvant treatment in patients with Dukes' C colon cancer is recommended for a total of 6 months [ie, capecitabine tablets 1250 mg/m 2 orally twice daily for 2 weeks followed by a 1-week rest period, given as 3-week cycles for a total of 8 cycles (24 weeks)].
| Dose Level 1250 mg/m
2
Twice a Day | Number of Tablets to be Taken at Each Dose (Morning and Evening) | ||
|---|---|---|---|
| Surface Area (m 2) | Total Daily Dose * (mg) | 150 mg | 500 mg |
|
|||
| ≤ 1.25 | 3000 | 0 | 3 |
| 1.26 to 1.37 | 3300 | 1 | 3 |
| 1.38 to 1.51 | 3600 | 2 | 3 |
| 1.52 to 1.65 | 4000 | 0 | 4 |
| 1.66 to 1.77 | 4300 | 1 | 4 |
| 1.78 to 1.91 | 4600 | 2 | 4 |
| 1.92 to 2.05 | 5000 | 0 | 5 |
| 2.06 to 2.17 | 5300 | 1 | 5 |
| ≥ 2.18 | 5600 | 2 | 5 |
In Combination With Docetaxel (Metastatic Breast Cancer)
In combination with docetaxel, the recommended dose of capecitabine tablets are 1250 mg/m 2 twice daily for 2 weeks followed by a 1-week rest period, combined with docetaxel at 75 mg/m 2 as a 1-hour intravenous infusion every 3 weeks. Pre-medication, according to the docetaxel labeling, should be started prior to docetaxel administration for patients receiving the capecitabine tablets plus docetaxel combination. Table 1 displays the total daily dose of capecitabine tablets by body surface area and the number of tablets to be taken at each dose.
2.3 Dose Management Guidelines
General
Capecitabine tablets dosage may need to be individualized to optimize patient management. Patients should be carefully monitored for toxicity and doses of capecitabine tablets should be modified as necessary to accommodate individual patient tolerance to treatment [see Clinical Studies (14)] . Toxicity due to capecitabine tablets administration may be managed by symptomatic treatment, dose interruptions and adjustment of capecitabine tablets dose. Once the dose has been reduced, it should not be increased at a later time. Doses of capecitabine tablets omitted for toxicity are not replaced or restored; instead the patient should resume the planned treatment cycles.
The dose of phenytoin and the dose of coumarin-derivative anticoagulants may need to be reduced when either drug is administered concomitantly with capecitabine tablets [see Drug Interactions (7.1)] .
Monotherapy (Metastatic Colorectal Cancer, Adjuvant Colorectal Cancer, Metastatic Breast Cancer)
Capecitabine tablets dose modification scheme as described below (see Table 2) is recommended for the management of adverse reactions.
| Toxicity NCIC Grades * |
During a Course of Therapy | Dose Adjustment for Next Treatment (% of starting dose) |
|---|---|---|
|
||
| Grade 1 | Maintain dose level | Maintain dose level |
| Grade 2 | ||
| -1st appearance | Interrupt until resolved to grade 0 to 1 | 100% |
| -2nd appearance | 75% | |
| -3rd appearance | 50% | |
| -4th appearance | Discontinue treatment permanently | - |
| Grade 3 | ||
| -1st appearance | Interrupt until resolved to grade 0 to 1 | 75% |
| -2nd appearance | 50% | |
| -3rd appearance | Discontinue treatment permanently | - |
| Grade 4 | ||
| -1st appearance | Discontinue permanently
OR If physician deems it to be in the patient's best interest to continue, interrupt until resolved to grade 0 to 1 | 50% |
In Combination With Docetaxel (Metastatic Breast Cancer)
Dose modifications of capecitabine tablets for toxicity should be made according to Table 2 above for capecitabine tablets. At the beginning of a treatment cycle, if a treatment delay is indicated for either capecitabine tablets or docetaxel, then administration of both agents should be delayed until the requirements for restarting both drugs are met.
The dose reduction schedule for docetaxel when used in combination with capecitabine tablets for the treatment of metastatic breast cancer is shown in Table 3.
| Toxicity NCIC Grades * | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
|
|||
| 1st appearance | Delay treatment until resolved to grade 0 to 1; Resume treatment with original dose of 75 mg/m 2 docetaxel | Delay treatment until resolved to grade 0 to 1;
Resume treatment at 55 mg/m2 of docetaxel. | Discontinue treatment with docetaxel |
| 2nd appearance | Delay treatment until resolved to grade 0 to 1; Resume treatment at 55 mg/m 2 of docetaxel. | Discontinue treatment with docetaxel | - |
| 3rd appearance | Discontinue treatment with docetaxel | - | - |
2.4 Adjustment of Starting Dose in Special Populations
Renal Impairment
No adjustment to the starting dose of capecitabine tablets are recommended in patients with mild renal impairment (creatinine clearance = 51 to 80 mL/min [Cockroft and Gault, as shown below]). In patients with moderate renal impairment (baseline creatinine clearance = 30 to 50 mL/min), a dose reduction to 75% of the capecitabine tablets starting dose when used as monotherapy or in combination with docetaxel (from 1250 mg/m 2 to 950 mg/m 2 twice daily) is recommended [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)] . Subsequent dose adjustment is recommended as outlined in Table 2 and Table 3 (depending on the regimen) if a patient develops a grade 2 to 4 adverse event [see Warnings and Precautions (5.5)] . The starting dose adjustment recommendations for patients with moderate renal impairment apply to both capecitabine tablets monotherapy and capecitabine tablets in combination use with docetaxel.
| Cockroft and Gault Equation: | |
| (140 - age [yrs]) (body wt [kg]) | |
| Creatinine clearance for males = | ————————————— |
| (72) (serum creatinine [mg/dL]) | |
| Creatinine clearance for females = 0.85 × male value | |
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Severe Renal Impairment
Capecitabine is contraindicated in patients with severe renal impairment (creatinine clearance below 30 mL/min [Cockroft and Gault]) [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)] .
5 WARNINGS AND PRECAUTIONS
5.1 Coagulopathy
Patients receiving concomitant capecitabine and oral coumarin-derivative anticoagulant therapy should have their anticoagulant response (INR or prothrombin time) monitored closely with great frequency and the anticoagulant dose should be adjusted accordingly [see Boxed Warning and Drug Interactions (7.1)]
5.2 Diarrhea
Capecitabine can induce diarrhea, sometimes severe. Patients with severe diarrhea should be carefully monitored and given fluid and electrolyte replacement if they become dehydrated. In 875 patients with either metastatic breast or colorectal cancer who received capecitabine monotherapy, the median time to first occurrence of grade 2 to 4 diarrhea was 34 days (range from 1 to 369 days). The median duration of grade 3 to 4 diarrhea was 5 days. National Cancer Institute of Canada (NCIC) grade 2 diarrhea is defined as an increase of 4 to 6 stools/day or nocturnal stools, grade 3 diarrhea as an increase of 7 to 9 stools/day or incontinence and malabsorption, and grade 4 diarrhea as an increase of ≥10 stools/day or grossly bloody diarrhea or the need for parenteral support. If grade 2, 3 or 4 diarrhea occurs, administration of capecitabine should be immediately interrupted until the diarrhea resolves or decreases in intensity to grade 1 [see Dosage and Administration (2.3)] . Standard antidiarrheal treatments (e.g., loperamide) are recommended.
Necrotizing enterocolitis (typhlitis) has been reported.
5.3 Cardiotoxicity
The cardiotoxicity observed with capecitabine includes myocardial infarction/ischemia, angina, dysrhythmias, cardiac arrest, cardiac failure, sudden death, electrocardiographic changes, and cardiomyopathy. These adverse reactions may be more common in patients with a prior history of coronary artery disease.
5.4 Dihydropyrimidine Dehydrogenase Deficiency
Based on postmarketing reports, patients with certain homozygous or certain compound heterozygous mutations in the DPD gene that result in complete or near complete absence of DPD activity are at increased risk for acute early-onset of toxicity and severe, life-threatening, or fatal adverse reactions caused by capecitabine (e.g., mucositis, diarrhea, neutropenia, and neurotoxicity). Patients with partial DPD activity may also have increased risk of severe, life-threatening, or fatal adverse reactions caused by capecitabine.
Withhold or permanently discontinue capecitabine based on clinical assessment of the onset, duration and severity of the observed toxicities in patients with evidence of acute early-onset or unusually severe toxicity, which may indicate near complete or total absence of DPD activity. No capecitabine dose has been proven safe for patients with complete absence of DPD activity. There is insufficient data to recommend a specific dose in patients with partial DPD activity as measured by any specific test.
5.5 Dehydration and Renal Failure
Dehydration has been observed and may cause acute renal failure which can be fatal. Patients with pre-existing compromised renal function or who are receiving concomitant capecitabine with known nephrotoxic agents are at higher risk. Patients with anorexia, asthenia, nausea, vomiting or diarrhea may rapidly become dehydrated. Monitor patients when capecitabine is administered to prevent and correct dehydration at the onset. If grade 2 (or higher) dehydration occurs, capecitabine treatment should be immediately interrupted and the dehydration corrected. Treatment should not be restarted until the patient is rehydrated and any precipitating causes have been corrected or controlled. Dose modifications should be applied for the precipitating adverse event as necessary [see Dosage and Administration (2.3)] .
Patients with moderate renal impairment at baseline require dose reduction [see Dosage and Administration (2.4)] . Patients with mild and moderate renal impairment at baseline should be carefully monitored for adverse reactions. Prompt interruption of therapy with subsequent dose adjustments is recommended if a patient develops a grade 2 to 4 adverse event as outlined in Table 2 [see Dosage and Administration (2.3), Use in Specific Populations (8.7), and Clinical Pharmacology (12.3)] .
5.6 Embryo-Fetal Toxicity
Based on findings from animal reproduction studies and its mechanism of action, capecitabine may cause fetal harm when given to a pregnant woman [see Clinical Pharmacology (12.1)] . Limited available data are not sufficient to inform use of capecitabine in pregnant women. In animal reproduction studies, administration of capecitabine to pregnant animals during the period of organogenesis caused embryolethality and teratogenicity in mice and embryolethality in monkeys at 0.2 and 0.6 times the exposure (AUC) in patients receiving the recommended dose respectively [see Use in Specific Populations (8.1)] . Apprise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of capecitabine [see Use in Specific Populations (8.3)] .
5.7 Mucocutaneous and Dermatologic Toxicity
Severe mucocutaneous reactions, some with fatal outcome, such as Stevens-Johnson syndrome and Toxic Epidermal Necrolysis (TEN) can occur in patients treated with capecitabine [see Adverse Reactions (6.4)] . Capecitabine should be permanently discontinued in patients who experience a severe mucocutaneous reaction possibly attributable to capecitabine treatment.
Hand-and-foot syndrome (palmar-plantar erythrodysesthesia or chemotherapy-induced acral erythema) is a cutaneous toxicity. Median time to onset was 79 days (range from 11 to 360 days) with a severity range of grades 1 to 3 for patients receiving capecitabine monotherapy in the metastatic setting. Grade 1 is characterized by any of the following: numbness, dysesthesia/paresthesia, tingling, painless swelling or erythema of the hands and/or feet and/or discomfort which does not disrupt normal activities. Grade 2 hand-and-foot syndrome is defined as painful erythema and swelling of the hands and/or feet and/or discomfort affecting the patient’s activities of daily living. Grade 3 hand-and-foot syndrome is defined as moist desquamation, ulceration, blistering or severe pain of the hands and/or feet and/or severe discomfort that causes the patient to be unable to work or perform activities of daily living. Persistent or severe hand-and-foot syndrome (grade 2 and above) can eventually lead to loss of fingerprints which could impact patient identification. If grade 2 or 3 hand-and-foot syndrome occurs, administration of capecitabine should be interrupted until the event resolves or decreases in intensity to grade 1. Following grade 3 hand-and-foot syndrome, subsequent doses of capecitabine should be decreased [see Dosage and Administration (2.3)] .
5.8 Hyperbilirubinemia
In 875 patients with either metastatic breast or colorectal cancer who received at least one dose of capecitabine 1250 mg/m 2 twice daily as monotherapy for 2 weeks followed by a 1-week rest period, grade 3 (1.5 to 3 × ULN) hyperbilirubinemia occurred in 15.2% (n=133) of patients and grade 4 (>3 × ULN) hyperbilirubinemia occurred in 3.9% (n=34) of patients. Of 566 patients who had hepatic metastases at baseline and 309 patients without hepatic metastases at baseline, grade 3 or 4 hyperbilirubinemia occurred in 22.8% and 12.3%, respectively. Of the 167 patients with grade 3 or 4 hyperbilirubinemia, 18.6% (n=31) also had postbaseline elevations (grades 1 to 4, without elevations at baseline) in alkaline phosphatase and 27.5% (n=46) had postbaseline elevations in transaminases at any time (not necessarily concurrent). The majority of these patients, 64.5% (n=20) and 71.7% (n=33), had liver metastases at baseline. In addition, 57.5% (n=96) and 35.3% (n=59) of the 167 patients had elevations (grades 1 to 4) at both prebaseline and postbaseline in alkaline phosphatase or transaminases, respectively. Only 7.8% (n=13) and 3.0% (n=5) had grade 3 or 4 elevations in alkaline phosphatase or transaminases.
In the 596 patients treated with capecitabine as first-line therapy for metastatic colorectal cancer, the incidence of grade 3 or 4 hyperbilirubinemia was similar to the overall clinical trial safety database of capecitabine monotherapy. The median time to onset for grade 3 or 4 hyperbilirubinemia in the colorectal cancer population was 64 days and median total bilirubin increased from 8 µm/L at baseline to 13 µm/L during treatment with capecitabine. Of the 136 colorectal cancer patients with grade 3 or 4 hyperbilirubinemia, 49 patients had grade 3 or 4 hyperbilirubinemia as their last measured value, of which 46 had liver metastases at baseline.
In 251 patients with metastatic breast cancer who received a combination of capecitabine and docetaxel, grade 3 (1.5 to 3 × ULN) hyperbilirubinemia occurred in 7% (n=17) and grade 4 (>3 × ULN) hyperbilirubinemia occurred in 2% (n=5).
If drug-related grade 3 to 4 elevations in bilirubin occur, administration of capecitabine should be immediately interrupted until the hyperbilirubinemia decreases to ≤3.0 X ULN [see recommended dose modifications under Dosage and Administration (2.3)] .
5.9 Hematologic
In 875 patients with either metastatic breast or colorectal cancer who received a dose of 1250 mg/m 2 administered twice daily as monotherapy for 2 weeks followed by a 1-week rest period, 3.2%, 1.7%, and 2.4% of patients had grade 3 or 4 neutropenia, thrombocytopenia or decreases in hemoglobin, respectively. In 251 patients with metastatic breast cancer who received a dose of capecitabine in combination with docetaxel, 68% had grade 3 or 4 neutropenia, 2.8% had grade 3 or 4 thrombocytopenia, and 9.6% had grade 3 or 4 anemia.
Patients with baseline neutrophil counts of <1.5 × 10 9/L and/or thrombocyte counts of <100 × 10 9/L should not be treated with capecitabine. If unscheduled laboratory assessments during a treatment cycle show grade 3 or 4 hematologic toxicity, treatment with capecitabine should be interrupted.
5.10 Geriatric Patients
Patients ≥80 years old may experience a greater incidence of grade 3 or 4 adverse reactions. In 875 patients with either metastatic breast or colorectal cancer who received capecitabine monotherapy, 62% of the 21 patients ≥80 years of age treated with capecitabine experienced a treatment-related grade 3 or 4 adverse event: diarrhea in 6 (28.6%), nausea in 3 (14.3%), hand-and-foot syndrome in 3 (14.3%), and vomiting in 2 (9.5%) patients. Among the 10 patients 70 years of age and greater (no patients were >80 years of age) treated with capecitabine in combination with docetaxel, 30% (3 out of 10) of patients experienced grade 3 or 4 diarrhea and stomatitis, and 40% (4 out of 10) experienced grade 3 hand-and-foot syndrome.
Among the 67 patients ≥60 years of age receiving capecitabine in combination with docetaxel, the incidence of grade 3 or 4 treatment-related adverse reactions, treatment-related serious adverse reactions, withdrawals due to adverse reactions, treatment discontinuations due to adverse reactions and treatment discontinuations within the first two treatment cycles was higher than in the <60 years of age patient group.
In 995 patients receiving capecitabine as adjuvant therapy for Dukes' C colon cancer after resection of the primary tumor, 41% of the 398 patients ≥65 years of age treated with capecitabine experienced a treatment-related grade 3 or 4 adverse event: hand-and-foot syndrome in 75 (18.8%), diarrhea in 52 (13.1%), stomatitis in 12 (3.0%), neutropenia/granulocytopenia in 11 (2.8%), vomiting in 6 (1.5%), and nausea in 5 (1.3%) patients. In patients ≥65 years of age (all randomized population; capecitabine 188 patients, 5-FU/LV 208 patients) treated for Dukes' C colon cancer after resection of the primary tumor, the hazard ratios for disease-free survival and overall survival for capecitabine compared to 5-FU/LV were 1.01 (95% C.I. 0.80 to 1.27) and 1.04 (95% C.I. 0.79 to 1.37), respectively.
5.11 Hepatic Insufficiency
Patients with mild to moderate hepatic dysfunction due to liver metastases should be carefully monitored when capecitabine is administered. The effect of severe hepatic dysfunction on the disposition of capecitabine is not known [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)] .
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Adjuvant Colon Cancer
Table 4 shows the adverse reactions occurring in ≥5% of patients from one phase 3 trial in patients with Dukes' C colon cancer who received at least one dose of study medication and had at least one safety assessment. A total of 995 patients were treated with 1250 mg/m 2 twice a day of capecitabine administered for 2 weeks followed by a 1-week rest period, and 974 patients were administered 5-FU and leucovorin (20 mg/m 2 leucovorin IV followed by 425 mg/m 2 IV bolus 5-FU on days 1 to 5 every 28 days). The median duration of treatment was 164 days for capecitabine-treated patients and 145 days for 5-FU/LV-treated patients. A total of 112 (11%) and 73 (7%) capecitabine and 5-FU/LV-treated patients, respectively, discontinued treatment because of adverse reactions. A total of 18 deaths due to all causes occurred either on study or within 28 days of receiving study drug: 8 (0.8%) patients randomized to capecitabine and 10 (1.0%) randomized to 5-FU/LV.
Table 5 shows grade 3/4 laboratory abnormalities occurring in ≥1% of patients from one phase 3 trial in patients with Dukes' C colon cancer who received at least one dose of study medication and had at least one safety assessment.
| Adjuvant Treatment for Colon Cancer (N=1969) | ||||
|---|---|---|---|---|
| Capecitabine
(N=995) | 5-FU/LV
(N=974) |
|||
| Body System/
Adverse Event | All Grades | Grade 3/4 | All Grades | Grade 3/4 |
| Gastrointestinal Disorders | ||||
| Diarrhea | 47 | 12 | 65 | 14 |
| Nausea | 34 | 2 | 47 | 2 |
| Stomatitis | 22 | 2 | 60 | 14 |
| Vomiting | 15 | 2 | 21 | 2 |
| Abdominal Pain | 14 | 3 | 16 | 2 |
| Constipation | 9 | - | 11 | <1 |
| Upper Abdominal Pain | 7 | <1 | 7 | <1 |
| Dyspepsia | 6 | <1 | 5 | - |
| Skin and Subcutaneous Tissue Disorders | ||||
| Hand-and-Foot Syndrome | 60 | 17 | 9 | <1 |
| Alopecia | 6 | - | 22 | <1 |
| Rash | 7 | - | 8 | - |
| Erythema | 6 | 1 | 5 | <1 |
| General Disorders and Administration Site Conditions | ||||
| Fatigue | 16 | <1 | 16 | 1 |
| Pyrexia | 7 | <1 | 9 | <1 |
| Asthenia | 10 | <1 | 10 | 1 |
| Lethargy | 10 | <1 | 9 | <1 |
| Nervous System Disorders | ||||
| Dizziness | 6 | <1 | 6 | - |
| Headache | 5 | <1 | 6 | <1 |
| Dysgeusia | 6 | - | 9 | - |
| Metabolism and Nutrition Disorders | ||||
| Anorexia | 9 | <1 | 11 | <1 |
| Eye Disorders | ||||
| Conjunctivitis | 5 | <1 | 6 | <1 |
| Blood and Lymphatic System Disorders | ||||
| Neutropenia | 2 | <1 | 8 | 5 |
| Respiratory Thoracic and Mediastinal Disorders | ||||
| Epistaxis | 2 | - | 5 | - |
| Adverse Event | Capecitabine
(n=995) Grade 3/4 % | IV 5-FU/LV
(n=974) Grade 3/4 % |
|---|---|---|
|
||
| Increased ALAT (SGPT) | 1.6 | 0.6 |
| Increased calcium | 1.1 | 0.7 |
| Decreased calcium | 2.3 | 2.2 |
| Decreased hemoglobin | 1.0 | 1.2 |
| Decreased lymphocytes | 13.0 | 13.0 |
| Decreased neutrophils * | 2.2 | 26.2 |
| Decreased neutrophils/granulocytes | 2.4 | 26.4 |
| Decreased platelets | 1.0 | 0.7 |
| Increased bilirubin † | 20 | 6.3 |
6.2 Metastatic Colorectal Cancer
Monotherapy
Table 6 shows the adverse reactions occurring in ≥5% of patients from pooling the two phase 3 trials in first line metastatic colorectal cancer. A total of 596 patients with metastatic colorectal cancer were treated with 1250 mg/m 2 twice a day of capecitabine administered for 2 weeks followed by a 1-week rest period, and 593 patients were administered 5-FU and leucovorin in the Mayo regimen (20 mg/m 2 leucovorin IV followed by 425 mg/m 2 IV bolus 5-FU, on days 1 to 5, every 28 days). In the pooled colorectal database the median duration of treatment was 139 days for capecitabine-treated patients and 140 days for 5-FU/LV-treated patients. A total of 78 (13%) and 63 (11%) capecitabine and 5-FU/LV-treated patients, respectively, discontinued treatment because of adverse reactions/intercurrent illness. A total of 82 deaths due to all causes occurred either on study or within 28 days of receiving study drug: 50 (8.4%) patients randomized to capecitabine and 32 (5.4%) randomized to 5-FU/LV.
| Adverse Event | Capecitabine
(n=596) | 5-FU/LV
(n=593) |
||||
|---|---|---|---|---|---|---|
| Total % | Grade 3 % | Grade 4 % | Total % | Grade 3 % | Grade 4 % | |
| – Not observed | ||||||
| NA = Not Applicable | ||||||
|
||||||
| Number of Patients With > One Adverse Event | 96 | 52 | 9 | 94 | 45 | 9 |
| Body System/Adverse Event | ||||||
| GI | ||||||
| Diarrhea | 55 | 13 | 2 | 61 | 10 | 2 |
| Nausea | 43 | 4 | – | 51 | 3 | <1 |
| Vomiting | 27 | 4 | <1 | 30 | 4 | <1 |
| Stomatitis | 25 | 2 | <1 | 62 | 14 | 1 |
| Abdominal Pain | 35 | 9 | <1 | 31 | 5 | – |
| Gastrointestinal Motility Disorder | 10 | <1 | – | 7 | <1 | – |
| Constipation | 14 | 1 | <1 | 17 | 1 | – |
| Oral Discomfort | 10 | – | – | 10 | – | – |
| Upper GI Inflammatory Disorders | 8 | <1 | – | 10 | 1 | – |
| Gastrointestinal Hemorrhage | 6 | 1 | <1 | 3 | 1 | – |
| Ileus | 6 | 4 | 1 | 5 | 2 | 1 |
| Skin and Subcutaneous | ||||||
| Hand-and-Foot Syndrome | 54 | 17 | NA | 6 | 1 | NA |
| Dermatitis | 27 | 1 | – | 26 | 1 | – |
| Skin Discoloration | 7 | <1 | – | 5 | – | – |
| Alopecia | 6 | – | – | 21 | <1 | – |
| General | ||||||
| Fatigue/Weakness | 42 | 4 | – | 46 | 4 | – |
| Pyrexia | 18 | 1 | – | 21 | 2 | – |
| Edema | 15 | 1 | – | 9 | 1 | – |
| Pain | 12 | 1 | – | 10 | 1 | – |
| Chest Pain | 6 | 1 | – | 6 | 1 | <1 |
| Neurological | ||||||
| Peripheral Sensory Neuropathy | 10 | – | – | 4 | – | – |
| Headache | 10 | 1 | – | 7 | – | – |
| Dizziness * | 8 | <1 | – | 8 | <1 | – |
| Insomnia | 7 | – | – | 7 | – | – |
| Taste Disturbance | 6 | 1 | – | 11 | <1 | 1 |
| Metabolism | ||||||
| Appetite Decreased | 26 | 3 | <1 | 31 | 2 | <1 |
| Dehydration | 7 | 2 | <1 | 8 | 3 | 1 |
| Eye | ||||||
| Eye Irritation | 13 | – | – | 10 | <1 | – |
| Vision Abnormal | 5 | – | – | 2 | – | – |
| Respiratory | ||||||
| Dyspnea | 14 | 1 | – | 10 | <1 | 1 |
| Cough | 7 | <1 | 1 | 8 | – | – |
| Pharyngeal Disorder | 5 | – | – | 5 | – | – |
| Epistaxis | 3 | <1 | – | 6 | – | – |
| Sore Throat | 2 | – | – | 6 | – | – |
| Musculoskeletal | ||||||
| Back Pain | 10 | 2 | – | 9 | <1 | – |
| Arthralgia | 8 | 1 | – | 6 | 1 | – |
| Vascular | ||||||
| Venous Thrombosis | 8 | 3 | <1 | 6 | 2 | – |
| Psychiatric | ||||||
| Mood Alteration | 5 | – | – | 6 | <1 | – |
| Depression | 5 | – | – | 4 | <1 | – |
| Infections | ||||||
| Viral | 5 | <1 | – | 5 | <1 | – |
| Blood and Lymphatic | ||||||
| Anemia | 80 | 2 | <1 | 79 | 1 | <1 |
| Neutropenia | 13 | 1 | 2 | 46 | 8 | 13 |
| Hepatobiliary | ||||||
| Hyperbilirubinemia | 48 | 18 | 5 | 17 | 3 | 3 |
6.3 Breast Cancer
In Combination with Docetaxel
The following data are shown for the combination study with capecitabine and docetaxel in patients with metastatic breast cancer in Table 7 and Table 8. In the capecitabine and docetaxel combination arm the treatment was capecitabine administered orally 1250 mg/m 2 twice daily as intermittent therapy (2 weeks of treatment followed by 1 week without treatment) for at least 6 weeks and docetaxel administered as a 1-hour intravenous infusion at a dose of 75 mg/m 2 on the first day of each 3-week cycle for at least 6 weeks. In the monotherapy arm docetaxel was administered as a 1-hour intravenous infusion at a dose of 100 mg/m 2 on the first day of each 3-week cycle for at least 6 weeks. The mean duration of treatment was 129 days in the combination arm and 98 days in the monotherapy arm. A total of 66 patients (26%) in the combination arm and 49 (19%) in the monotherapy arm withdrew from the study because of adverse reactions. The percentage of patients requiring dose reductions due to adverse reactions was 65% in the combination arm and 36% in the monotherapy arm. The percentage of patients requiring treatment interruptions due to adverse reactions in the combination arm was 79%. Treatment interruptions were part of the dose modification scheme for the combination therapy arm but not for the docetaxel monotherapy-treated patients.
| Adverse Event | Capecitabine 1250 mg/m
2/bid
With Docetaxel 75 mg/m 2/3 weeks (n=251) | Docetaxel
100 mg/m 2/3 weeks (n=255) |
||||
|---|---|---|---|---|---|---|
| Total % | Grade 3 % | Grade 4 % | Total % | Grade 3 % | Grade 4 % | |
| – Not observed | ||||||
| NA = Not Applicable | ||||||
| Number of Patients With at Least One Adverse Event | 99 | 76.5 | 29.1 | 97 | 57.6 | 31.8 |
| Body System/Adverse Event | ||||||
| GI | ||||||
| Diarrhea | 67 | 14 | <1 | 48 | 5 | <1 |
| Stomatitis | 67 | 17 | <1 | 43 | 5 | – |
| Nausea | 45 | 7 | – | 36 | 2 | – |
| Vomiting | 35 | 4 | 1 | 24 | 2 | – |
| Constipation | 20 | 2 | – | 18 | – | – |
| Abdominal Pain | 30 | <3 | <1 | 24 | 2 | – |
| Dyspepsia | 14 | – | – | 8 | 1 | – |
| Dry Mouth | 6 | <1 | – | 5 | – | – |
| Skin and Subcutaneous | ||||||
| Hand-and-Foot Syndrome | 63 | 24 | NA | 8 | 1 | NA |
| Alopecia | 41 | 6 | – | 42 | 7 | – |
| Nail Disorder | 14 | 2 | – | 15 | – | – |
| Dermatitis | 8 | – | – | 11 | 1 | – |
| Rash Erythematous | 9 | <1 | – | 5 | – | – |
| Nail Discoloration | 6 | – | – | 4 | <1 | – |
| Onycholysis | 5 | 1 | – | 5 | 1 | – |
| Pruritus | 4 | – | – | 5 | – | – |
| General | ||||||
| Pyrexia | 28 | 2 | – | 34 | 2 | – |
| Asthenia | 26 | 4 | <1 | 25 | 6 | – |
| Fatigue | 22 | 4 | – | 27 | 6 | – |
| Weakness | 16 | 2 | – | 11 | 2 | – |
| Pain in Limb | 13 | <1 | – | 13 | 2 | – |
| Lethargy | 7 | – | – | 6 | 2 | – |
| Pain | 7 | <1 | – | 5 | 1 | – |
| Chest Pain (non-cardiac) | 4 | <1 | – | 6 | 2 | – |
| Influenza-like Illness | 5 | – | – | 5 | – | – |
| Neurological | ||||||
| Taste Disturbance | 16 | <1 | – | 14 | <1 | – |
| Headache | 15 | 3 | – | 15 | 2 | – |
| Paresthesia | 12 | <1 | – | 16 | 1 | – |
| Dizziness | 12 | – | – | 8 | <1 | – |
| Insomnia | 8 | – | – | 10 | <1 | – |
| Peripheral Neuropathy | 6 | – | – | 10 | 1 | – |
| Hypoaesthesia | 4 | <1 | – | 8 | <1 | – |
| Metabolism | ||||||
| Anorexia | 13 | 1 | – | 11 | <1 | – |
| Appetite Decreased | 10 | – | – | 5 | – | – |
| Weight Decreased | 7 | – | – | 5 | – | – |
| Dehydration | 10 | 2 | – | 7 | <1 | <1 |
| Eye | ||||||
| Lacrimation Increased | 12 | – | – | 7 | <1 | – |
| Conjunctivitis | 5 | – | – | 4 | – | – |
| Eye Irritation | 5 | – | – | 1 | – | – |
| Musculoskeletal | ||||||
| Arthralgia | 15 | 2 | – | 24 | 3 | – |
| Myalgia | 15 | 2 | – | 25 | 2 | – |
| Back Pain | 12 | <1 | – | 11 | 3 | – |
| Bone Pain | 8 | <1 | – | 10 | 2 | – |
| Cardiac | ||||||
| Edema | 33 | <2 | – | 34 | <3 | 1 |
| Blood | ||||||
| Neutropenic Fever | 16 | 3 | 13 | 21 | 5 | 16 |
| Respiratory | ||||||
| Dyspnea | 14 | 2 | <1 | 16 | 2 | – |
| Cough | 13 | 1 | – | 22 | <1 | – |
| Sore Throat | 12 | 2 | – | 11 | <1 | – |
| Epistaxis | 7 | <1 | – | 6 | – | – |
| Rhinorrhea | 5 | – | – | 3 | – | – |
| Pleural Effusion | 2 | 1 | – | 7 | 4 | – |
| Infection | ||||||
| Oral Candidiasis | 7 | <1 | – | 8 | <1 | – |
| Urinary Tract Infection | 6 | <1 | – | 4 | – | – |
| Upper Respiratory Tract | 4 | – | – | 5 | 1 | – |
| Vascular | ||||||
| Flushing | 5 | – | – | 5 | – | – |
| Lymphoedema | 3 | <1 | – | 5 | 1 | – |
| Psychiatric | ||||||
| Depression | 5 | – | – | 5 | 1 | – |
| Adverse Event | Capecitabine 1250 mg/m
2/bid
With Docetaxel 75 mg/m 2/3 weeks (n=251) | Docetaxel
100 mg/m 2/3 weeks (n=255) |
||||
|---|---|---|---|---|---|---|
| Body System/Adverse Event | Total % | Grade 3 % | Grade 4 % | Total % | Grade 3 % | Grade 4 % |
| Hematologic | ||||||
| Leukopenia | 91 | 37 | 24 | 88 | 42 | 33 |
| Neutropenia/Granulocytopenia | 86 | 20 | 49 | 87 | 10 | 66 |
| Thrombocytopenia | 41 | 2 | 1 | 23 | 1 | 2 |
| Anemia | 80 | 7 | 3 | 83 | 5 | <1 |
| Lymphocytopenia | 99 | 48 | 41 | 98 | 44 | 40 |
| Hepatobiliary | ||||||
| Hyperbilirubinemia | 20 | 7 | 2 | 6 | 2 | 2 |
Monotherapy
The following data are shown for the study in stage IV breast cancer patients who received a dose of 1250 mg/m 2 administered twice daily for 2 weeks followed by a 1-week rest period. The mean duration of treatment was 114 days. A total of 13 out of 162 patients (8%) discontinued treatment because of adverse reactions/intercurrent illness.
| Adverse Event | Phase 2 Trial in Stage IV Breast Cancer
(n=162) |
||
|---|---|---|---|
| Body System/Adverse Event | Total % | Grade 3 % | Grade 4 % |
| – Not observed | |||
| NA = Not Applicable | |||
| GI | |||
| Diarrhea | 57 | 12 | 3 |
| Nausea | 53 | 4 | – |
| Vomiting | 37 | 4 | – |
| Stomatitis | 24 | 7 | – |
| Abdominal Pain | 20 | 4 | – |
| Constipation | 15 | 1 | – |
| Dyspepsia | 8 | – | – |
| Skin and Subcutaneous | |||
| Hand-and-Foot Syndrome | 57 | 11 | NA |
| Dermatitis | 37 | 1 | – |
| Nail Disorder | 7 | – | – |
| General | |||
| Fatigue | 41 | 8 | – |
| Pyrexia | 12 | 1 | – |
| Pain in Limb | 6 | 1 | – |
| Neurological | |||
| Paresthesia | 21 | 1 | – |
| Headache | 9 | 1 | – |
| Dizziness | 8 | – | – |
| Insomnia | 8 | – | – |
| Metabolism | |||
| Anorexia | 23 | 3 | – |
| Dehydration | 7 | 4 | 1 |
| Eye | |||
| Eye Irritation | 15 | – | – |
| Musculoskeletal | |||
| Myalgia | 9 | – | – |
| Cardiac | |||
| Edema | 9 | 1 | – |
| Blood | |||
| Neutropenia | 26 | 2 | 2 |
| Thrombocytopenia | 24 | 3 | 1 |
| Anemia | 72 | 3 | 1 |
| Lymphopenia | 94 | 44 | 15 |
| Hepatobiliary | |||
| Hyperbilirubinemia | 22 | 9 | 2 |
6.4 Clinically Relevant Adverse Events in <5% of Patients
Clinically relevant adverse events reported in <5% of patients treated with capecitabine either as monotherapy or in combination with docetaxel that were considered at least remotely related to treatment are shown below; occurrences of each grade 3 and 4 adverse event are provided in parentheses.
Monotherapy (Metastatic Colorectal Cancer, Adjuvant Colorectal Cancer, Metastatic Breast Cancer)
| Gastrointestinal: | abdominal distension, dysphagia, proctalgia, ascites (0.1%), gastric ulcer (0.1%), ileus (0.3%), toxic dilation of intestine, gastroenteritis (0.1%) |
| Skin & Subcutan.: | nail disorder (0.1%), sweating increased (0.1%), photosensitivity reaction (0.1%), skin ulceration, pruritus, radiation recall syndrome (0.2%) |
| General: | chest pain (0.2%), influenza-like illness, hot flushes, pain (0.1%), hoarseness, irritability, difficulty in walking, thirst, chest mass, collapse, fibrosis (0.1%), hemorrhage, edema, sedation |
| Neurological: | insomnia, ataxia (0.5%), tremor, dysphasia, encephalopathy (0.1%), abnormal coordination, dysarthria, loss of consciousness (0.2%), impaired balance |
| Metabolism: | increased weight, cachexia (0.4%), hypertriglyceridemia (0.1%), hypokalemia, hypomagnesemia |
| Eye: | conjunctivitis |
| Respiratory: | cough (0.1%), epistaxis (0.1%), asthma (0.2%), hemoptysis, respiratory distress (0.1%), dyspnea |
| Cardiac: | tachycardia (0.1%), bradycardia, atrial fibrillation, ventricular extrasystoles, extrasystoles, myocarditis (0.1%), pericardial effusion |
| Infections: | laryngitis (1.0%), bronchitis (0.2%), pneumonia (0.2%), bronchopneumonia (0.2%), keratoconjunctivitis, sepsis (0.3%), fungal infections (including candidiasis) (0.2%) |
| Musculoskeletal: | myalgia, bone pain (0.1%), arthritis (0.1%), muscle weakness |
| Blood & Lymphatic: | leukopenia (0.2%), coagulation disorder (0.1%), bone marrow depression (0.1%), idiopathic thrombocytopenia purpura (1.0%), pancytopenia (0.1%) |
| Vascular: | hypotension (0.2%), hypertension (0.1%), lymphoedema (0.1%), pulmonary embolism (0.2%), cerebrovascular accident (0.1%) |
| Psychiatric: | depression, confusion (0.1%) |
| Renal: | renal impairment (0.6%) |
| Ear: | vertigo |
| Hepatobiliary: | hepatic fibrosis (0.1%), hepatitis (0.1%), cholestatic hepatitis (0.1%), abnormal liver function tests |
| Immune System: | drug hypersensitivity (0.1%) |
Capecitabine In Combination With Docetaxel (Metastatic Breast Cancer)
| Gastrointestinal: | ileus (0.4%), necrotizing enterocolitis (0.4%), esophageal ulcer (0.4%), hemorrhagic diarrhea (0.8%) |
| Neurological: | ataxia (0.4%), syncope (1.2%), taste loss (0.8%), polyneuropathy (0.4%), migraine (0.4%) |
| Cardiac: | supraventricular tachycardia (0.4%) |
| Infection: | neutropenic sepsis (2.4%), sepsis (0.4%), bronchopneumonia (0.4%) |
| Blood & Lymphatic: | agranulocytosis (0.4%), prothrombin decreased (0.4%) |
| Vascular: | hypotension (1.2%), venous phlebitis and thrombophlebitis (0.4%), postural hypotension (0.8%) |
| Renal: | renal failure (0.4%) |
| Hepatobiliary: | jaundice (0.4%), abnormal liver function tests (0.4%), hepatic failure (0.4%), hepatic coma (0.4%), hepatotoxicity (0.4%) |
| Immune System: | hypersensitivity (1.2%) |
6.5 Postmarketing Experience
The following adverse reactions have been observed in the postmarketing setting: angioedema, lacrimal duct stenosis, acute renal failure secondary to dehydration including fatal outcome [see Warnings and Precautions (5.5)] , cutaneous lupus erythematosus, corneal disorders including keratitis, toxic leukoencephalopathy, severe skin reactions such as Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis (TEN) [see Warnings and Precautions (5.7)] , persistent or severe hand-and-foot syndrome can eventually lead to loss of fingerprints [see Warnings and Precautions (5.7)]
In instances of exposure to crushed capecitabine tablets, the following adverse reactions have been reported: eye irritation and swelling, skin rash, diarrhea, paresthesia, headache, gastric irritation, vomiting, and nausea.
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
Anticoagulants
Altered coagulation parameters and/or bleeding have been reported in patients taking capecitabine concomitantly with coumarin-derivative anticoagulants such as warfarin and phenprocoumon [see Boxed Warning] . These events occurred within several days and up to several months after initiating capecitabine therapy and, in a few cases, within 1 month after stopping capecitabine. These events occurred in patients with and without liver metastases. In a drug interaction study with single-dose warfarin administration, there was a significant increase in the mean AUC of S-warfarin [see Clinical Pharmacology (12.3)] . The maximum observed INR value increased by 91%. This interaction is probably due to an inhibition of cytochrome P450 2C9 by capecitabine and/or its metabolites.
Phenytoin
The level of phenytoin should be carefully monitored in patients taking capecitabine and phenytoin dose may need to be reduced [see Dosage and Administration (2.3)] . Postmarketing reports indicate that some patients receiving capecitabine and phenytoin had toxicity associated with elevated phenytoin levels. Formal drug-drug interaction studies with phenytoin have not been conducted, but the mechanism of interaction is presumed to be inhibition of the CYP2C9 isoenzyme by capecitabine and/or its metabolites.
Leucovorin
The concentration of 5-fluorouracil is increased and its toxicity may be enhanced by leucovorin. Deaths from severe enterocolitis, diarrhea, and dehydration have been reported in elderly patients receiving weekly leucovorin and fluorouracil.
CYP2C9 substrates
Other than warfarin, no formal drug-drug interaction studies between capecitabine and other CYP2C9 substrates have been conducted. Care should be exercised when capecitabine is coadministered with CYP2C9 substrates.
Allopurinol
Concomitant use with allopurinol may decrease concentration of capecitabine’s active metabolites [see Clinical Pharmacology (12.3)] , which may decrease capecitabine efficacy. Avoid the use of allopurinol during treatment with capecitabine.
7.2 Drug-Food Interaction
Food was shown to reduce both the rate and extent of absorption of capecitabine [see Clinical Pharmacology (12.3)] . In all clinical trials, patients were instructed to administer capecitabine within 30 minutes after a meal. It is recommended that capecitabine be administered with food [see Dosage and Administration (2)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal reproduction studies and its mechanism of action, capecitabine can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Limited available human data are not sufficient to inform the drug-associated risk during pregnancy. In animal reproduction studies, administration of capecitabine to pregnant animals during the period of organogenesis caused embryo lethality and teratogenicity in mice and embryo lethality in monkeys at 0.2 and 0.6 times the exposure (AUC) in patients receiving the recommended dose respectively [see Data]. Apprise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Oral administration of capecitabine to pregnant mice during the period of organogenesis at a dose of 198 mg/kg/day caused malformations and embryo lethality. In separate pharmacokinetic studies, this dose in mice produced 5’-DFUR AUC values that were approximately 0.2 times the AUC values in patients administered the recommended daily dose. Malformations in mice included cleft palate, anophthalmia, microphthalmia, oligodactyly, polydactyly, syndactyly, kinky tail and dilation of cerebral ventricles. Oral administration of capecitabine to pregnant monkeys during the period of organogenesis at a dose of 90 mg/kg/day, caused fetal lethality. This dose produced 5’-DFUR AUC values that were approximately 0.6 times the AUC values in patients administered the recommended daily dose.
8.2 Lactation
Risk Summary
There is no information regarding the presence of capecitabine in human milk, or on its effects on milk production or the breast-fed infant. Capecitabine metabolites were present in the milk of lactating mice [see Data]. Because of the potential for serious adverse reactions from capecitabine exposure in breast-fed infants, advise women not to breastfeed during treatment with capecitabine and for 2 weeks after the final dose.
Data
Lactating mice given a single oral dose of capecitabine excreted significant amounts of capecitabine metabolites into the milk.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiating capecitabine.
Contraception
Females
Capecitabine can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)] . Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the final dose of capecitabine.
Males
Based on genetic toxicity findings, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months following the final dose of capecitabine [see Nonclinical Toxicology (13.1)] .
Infertility
Based on animal studies, capecitabine may impair fertility in females and males of reproductive potential [see Nonclinical Toxicology (13.1)] .
8.4 Pediatric Use
The safety and effectiveness of capecitabine in pediatric patients have not been established. No clinical benefit was demonstrated in two single arm trials in pediatric patients with newly diagnosed brainstem gliomas and high grade gliomas. In both trials, pediatric patients received an investigational pediatric formulation of capecitabine concomitantly with and following completion of radiation therapy (total dose of 5580 cGy in 180 cGy fractions). The relative bioavailability of the investigational formulation to capecitabine was similar.
The first trial was conducted in 22 pediatric patients (median age 8 years, range 5 to 17 years) with newly diagnosed non-disseminated intrinsic diffuse brainstem gliomas and high grade gliomas. In the dose-finding portion of the trial, patients received capecitabine with concomitant radiation therapy at doses ranging from 500 mg/m 2 to 850 mg/m 2 every 12 hours for up to 9 weeks. After a 2 week break, patients received 1250 mg/m 2 capecitabine every 12 hours on Days 1 to 14 of a 21-day cycle for up to 3 cycles. The maximum tolerated dose (MTD) of capecitabine administered concomitantly with radiation therapy was 650 mg/m 2 every 12 hours. The major dose limiting toxicities were palmar-plantar erythrodysesthesia and alanine aminotransferase (ALT) elevation.
The second trial was conducted in 34 additional pediatric patients with newly diagnosed non-disseminated intrinsic diffuse brainstem gliomas (median age 7 years, range 3 to 16 years) and 10 pediatric patients who received the MTD of capecitabine in the dose-finding trial and met the eligibility criteria for this trial. All patients received 650 mg/m 2 capecitabine every 12 hours with concomitant radiation therapy for up to 9 weeks. After a 2 week break, patients received 1250 mg/m 2 capecitabine every 12 hours on Days 1-14 of a 21-day cycle for up to 3 cycles.
There was no improvement in one-year progression-free survival rate and one-year overall survival rate in pediatric patients with newly diagnosed intrinsic brainstem gliomas who received capecitabine relative to a similar population of pediatric patients who participated in other clinical trials.
The adverse reaction profile of capecitabine was consistent with the known adverse reaction profile in adults, with the exception of laboratory abnormalities which occurred more commonly in pediatric patients. The most frequently reported laboratory abnormalities (per-patient incidence ≥40%) were increased ALT (75%), lymphocytopenia (73%), leukopenia (73%), hypokalemia (68%), thrombocytopenia (57%), hypoalbuminemia (55%), neutropenia (50%), low hematocrit (50%), hypocalcemia (48%), hypophosphatemia (45%) and hyponatremia (45%).
8.5 Geriatric Use
Physicians should pay particular attention to monitoring the adverse effects of capecitabine in the elderly [see Warnings and Precautions (5.10)] .
8.6 Hepatic Insufficiency
Exercise caution when patients with mild to moderate hepatic dysfunction due to liver metastases are treated with capecitabine. The effect of severe hepatic dysfunction on capecitabine is not known [see Warnings and Precautions (5.11) and Clinical Pharmacology (12.3)].
8.7 Renal Insufficiency
Patients with moderate (creatinine clearance = 30 to 50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment showed higher exposure for capecitabine, 5-DFUR, and FBAL than in those with normal renal function [see Contraindications (4.2), Warnings and Precautions (5.5), Dosage and Administration (2.4), and Clinical Pharmacology (12.3)] .
10 OVERDOSAGE
The manifestations of acute overdose would include nausea, vomiting, diarrhea, gastrointestinal irritation and bleeding, and bone marrow depression. Medical management of overdose should include customary supportive medical interventions aimed at correcting the presenting clinical manifestations. Although no clinical experience using dialysis as a treatment for capecitabine overdose has been reported, dialysis may be of benefit in reducing circulating concentrations of 5'-DFUR, a low–molecular-weight metabolite of the parent compound.
Single doses of capecitabine were not lethal to mice, rats, and monkeys at doses up to 2000 mg/kg (2.4, 4.8, and 9.6 times the recommended human daily dose on a mg/m 2 basis).
11 DESCRIPTION
Capecitabine is a fluoropyrimidine carbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5'-deoxy-5-fluorouridine (5'-DFUR) which is converted to 5-fluorouracil.
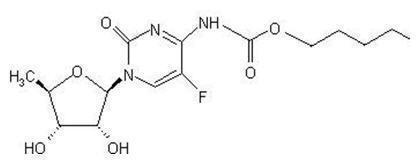
The chemical name for capecitabine is 5'-deoxy-5-fluoro-N-[(pentyloxy) carbonyl]-cytidine and has a molecular weight of 359.35. Capecitabine has the following structural formula:
Capecitabine is a white to off-white crystalline powder with an aqueous solubility of 26 mg/mL at 20°C.
Capecitabine tablets, USP are supplied as biconvex, oblong film-coated tablets for oral administration. Each light peach-colored tablet contains 150 mg capecitabine and each peach-colored tablet contains 500 mg capecitabine. The inactive ingredients in capecitabine tablets include: anhydrous lactose, croscarmellose sodium, hypromellose, microcrystalline cellulose and magnesium stearate. The peach or light peach film coating contains hypromellose, purified talc, titanium dioxide and ferric oxide red and ferric oxide yellow.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Enzymes convert capecitabine to 5-fluorouracil (5-FU) in vivo. Both normal and tumor cells metabolize 5-FU to 5-fluoro-2'-deoxyuridine monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). These metabolites cause cell injury by two different mechanisms. First, FdUMP and the folate cofactor, N 5-10-methylenetetrahydrofolate, bind to thymidylate synthase (TS) to form a covalently bound ternary complex. This binding inhibits the formation of thymidylate from 2'-deoxyuridylate. Thymidylate is the necessary precursor of thymidine triphosphate, which is essential for the synthesis of DNA, so that a deficiency of this compound can inhibit cell division. Second, nuclear transcriptional enzymes can mistakenly incorporate FUTP in place of uridine triphosphate (UTP) during the synthesis of RNA. This metabolic error can interfere with RNA processing and protein synthesis.
12.3 Pharmacokinetics
Absorption
Following oral administration of 1255 mg/m 2 BID to cancer patients, capecitabine reached peak blood levels in about 1.5 hours (T max) with peak 5-FU levels occurring slightly later, at 2 hours. Food reduced both the rate and extent of absorption of capecitabine with mean C max and AUC 0-∞ decreased by 60% and 35%, respectively. The C max and AUC 0-∞ of 5-FU were also reduced by food by 43% and 21%, respectively. Food delayed T max of both parent and 5-FU by 1.5 hours [see Warnings and Precautions (5), Dosage and Administration (2), and Drug-Food Interaction (7.2)] .
The pharmacokinetics of capecitabine and its metabolites have been evaluated in about 200 cancer patients over a dosage range of 500 to 3500 mg/m 2/day. Over this range, the pharmacokinetics of capecitabine and its metabolite, 5'-DFCR were dose proportional and did not change over time. The increases in the AUCs of 5'-DFUR and 5-FU, however, were greater than proportional to the increase in dose and the AUC of 5-FU was 34% higher on day 14 than on day 1. The interpatient variability in the C max and AUC of 5-FU was greater than 85%.
Distribution
Plasma protein binding of capecitabine and its metabolites is less than 60% and is not concentration-dependent. Capecitabine was primarily bound to human albumin (approximately 35%). Capecitabine has a low potential for pharmacokinetic interactions related to plasma protein binding.
Bioactivation and Metabolism
Capecitabine is extensively metabolized enzymatically to 5-FU. In the liver, a 60 kDa carboxylesterase hydrolyzes much of the compound to 5'-deoxy-5-fluorocytidine (5'-DFCR). Cytidine deaminase, an enzyme found in most tissues, including tumors, subsequently converts 5'-DFCR to 5'-DFUR. The enzyme, thymidine phosphorylase (dThdPase), then hydrolyzes 5'-DFUR to the active drug 5-FU. Many tissues throughout the body express thymidine phosphorylase. Some human carcinomas express this enzyme in higher concentrations than surrounding normal tissues. Following oral administration of capecitabine 7 days before surgery in patients with colorectal cancer, the median ratio of 5-FU concentration in colorectal tumors to adjacent tissues was 2.9 (range from 0.9 to 8.0). These ratios have not been evaluated in breast cancer patients or compared to 5-FU infusion.
 |
The enzyme dihydropyrimidine dehydrogenase hydrogenates 5-FU, the product of capecitabine metabolism, to the much less toxic 5-fluoro-5, 6-dihydro-fluorouracil (FUH 2). Dihydropyrimidinase cleaves the pyrimidine ring to yield 5-fluoro-ureido-propionic acid (FUPA). Finally, β-ureido-propionase cleaves FUPA to α-fluoro-β-alanine (FBAL) which is cleared in the urine.
In vitro enzymatic studies with human liver microsomes indicated that capecitabine and its metabolites (5'-DFUR, 5'-DFCR, 5-FU, and FBAL) did not inhibit the metabolism of test substrates by cytochrome P450 isoenzymes 1A2, 2A6, 3A4, 2C19, 2D6, and 2E1.
Excretion
Capecitabine and its metabolites are predominantly excreted in urine; 95.5% of administered capecitabine dose is recovered in urine. Fecal excretion is minimal (2.6%). The major metabolite excreted in urine is FBAL which represents 57% of the administered dose. About 3% of the administered dose is excreted in urine as unchanged drug. The elimination half-life of both parent capecitabine and 5-FU was about 0.75 hour.
Effect of Age, Gender, and Race on the Pharmacokinetics of Capecitabine
A population analysis of pooled data from the two large controlled studies in patients with metastatic colorectal cancer (n=505) who were administered capecitabine at 1250 mg/m 2 twice a day indicated that gender (202 females and 303 males) and race (455 white/Caucasian patients, 22 black patients, and 28 patients of other race) have no influence on the pharmacokinetics of 5'-DFUR, 5-FU and FBAL. Age has no significant influence on the pharmacokinetics of 5'-DFUR and 5-FU over the range of 27 to 86 years. A 20% increase in age results in a 15% increase in AUC of FBAL [see Warnings and Precautions (5.11) and Dosage and Administration (2.4)] .
Following oral administration of 825 mg/m 2 capecitabine twice daily for 14 days, Japanese patients (n=18) had about 36% lower C max and 24% lower AUC for capecitabine than the Caucasian patients (n=22). Japanese patients had also about 25% lower C max and 34% lower AUC for FBAL than the Caucasian patients. The clinical significance of these differences is unknown. No significant differences occurred in the exposure to other metabolites (5'-DFCR, 5'-DFUR, and 5-FU).
Effect of Hepatic Insufficiency
Capecitabine has been evaluated in 13 patients with mild to moderate hepatic dysfunction due to liver metastases defined by a composite score including bilirubin, AST/ALT and alkaline phosphatase following a single 1255 mg/m 2 dose of capecitabine. Both AUC 0-∞ and C max of capecitabine increased by 60% in patients with hepatic dysfunction compared to patients with normal hepatic function (n=14). The AUC 0-∞ and C max of 5-FU were not affected. In patients with mild to moderate hepatic dysfunction due to liver metastases, caution should be exercised when capecitabine is administered. The effect of severe hepatic dysfunction on capecitabine is not known [see Warnings and Precautions (5.11) and Use in Special Populations (8.6)].
Effect of Renal Insufficiency
Following oral administration of 1250 mg/m 2 capecitabine twice a day to cancer patients with varying degrees of renal impairment, patients with moderate (creatinine clearance = 30 to 50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment showed 85% and 258% higher systemic exposure to FBAL on day 1 compared to normal renal function patients (creatinine clearance >80 mL/min). Systemic exposure to 5'-DFUR was 42% and 71% greater in moderately and severely renal impaired patients, respectively, than in normal patients. Systemic exposure to capecitabine was about 25% greater in both moderately and severely renal impaired patients [see Dosage and Administration (2.4), Contraindications (4.2), Warnings and Precautions (5.5), and Use in Special Populations (8.7)] .
Effect of Capecitabine on the Pharmacokinetics of Warfarin
In four patients with cancer, chronic administration of capecitabine (1250 mg/m 2 bid) with a single 20 mg dose of warfarin increased the mean AUC of S-warfarin by 57% and decreased its clearance by 37%. Baseline corrected AUC of INR in these 4 patients increased by 2.8-fold, and the maximum observed mean INR value was increased by 91% [see Boxed Warning and Drug Interactions (7.1)].
Effect of Antacids on the Pharmacokinetics of Capecitabine
When Maalox® (20 mL), an aluminum hydroxide- and magnesium hydroxide-containing antacid, was administered immediately after capecitabine (1250 mg/m 2, n=12 cancer patients), AUC and C max increased by 16% and 35%, respectively, for capecitabine and by 18% and 22%, respectively, for 5'-DFCR. No effect was observed on the other three major metabolites (5'-DFUR, 5-FU, FBAL) of capecitabine.
Effect of Allopurinol on Capecitabine
Published literature reported that concomitant use with allopurinol may decrease conversion of capecitabine to the active metabolites, FdUMP and FUTP; however, the clinical significance was not fully characterized.
Effect of Capecitabine on the Pharmacokinetics of Docetaxel and Vice Versa
A Phase 1 study evaluated the effect of capecitabine on the pharmacokinetics of docetaxel (Taxotere ®) and the effect of docetaxel on the pharmacokinetics of capecitabine was conducted in 26 patients with solid tumors. Capecitabine was found to have no effect on the pharmacokinetics of docetaxel (C max and AUC) and docetaxel has no effect on the pharmacokinetics of capecitabine and the 5-FU precursor 5'-DFUR.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate studies investigating the carcinogenic potential of capecitabine have not been conducted. Capecitabine was not mutagenic in vitro to bacteria (Ames test) or mammalian cells (Chinese hamster V79/HPRT gene mutation assay). Capecitabine was clastogenic in vitro to human peripheral blood lymphocytes but not clastogenic in vivo to mouse bone marrow (micronucleus test). Fluorouracil causes mutations in bacteria and yeast. Fluorouracil also causes chromosomal abnormalities in the mouse micronucleus test in vivo.
In studies of fertility and general reproductive performance in female mice, oral capecitabine doses of 760 mg/kg/day (about 2300 mg/m 2/day) disturbed estrus and consequently caused a decrease in fertility. In mice that became pregnant, no fetuses survived this dose. The disturbance in estrus was reversible. In males, this dose caused degenerative changes in the testes, including decreases in the number of spermatocytes and spermatids. In separate pharmacokinetic studies, this dose in mice produced 5'-DFUR AUC values about 0.7 times the corresponding values in patients administered the recommended daily dose.
14 CLINICAL STUDIES
14.1 Adjuvant Colon Cancer
A multicenter randomized, controlled phase 3 clinical trial in patients with Dukes' C colon cancer (X-ACT) provided data concerning the use of capecitabine for the adjuvant treatment of patients with colon cancer. The primary objective of the study was to compare disease-free survival (DFS) in patients receiving capecitabine to those receiving IV 5-FU/LV alone. In this trial, 1987 patients were randomized either to treatment with capecitabine 1250 mg/m 2 orally twice daily for 2 weeks followed by a 1-week rest period, given as 3-week cycles for a total of 8 cycles (24 weeks) or IV bolus 5-FU 425 mg/m 2 and 20 mg/m 2 IV leucovorin on days 1 to 5, given as 4-week cycles for a total of 6 cycles (24 weeks). Patients in the study were required to be between 18 and 75 years of age with histologically-confirmed Dukes' stage C colon cancer with at least one positive lymph node and to have undergone (within 8 weeks prior to randomization) complete resection of the primary tumor without macroscopic or microscopic evidence of remaining tumor. Patients were also required to have no prior cytotoxic chemotherapy or immunotherapy (except steroids), and have an ECOG performance status of 0 or 1 (KPS ≥ 70%), ANC ≥ 1.5×10 9/L, platelets ≥ 100×10 9/L, serum creatinine ≤ 1.5 ULN, total bilirubin ≤ 1.5 ULN, AST/ALT ≤ 2.5 ULN and CEA within normal limits at time of randomization.
The baseline demographics for capecitabine and 5-FU/LV patients are shown in Table 10. The baseline characteristics were well-balanced between arms.
| Capecitabine
(n=1004) | 5-FU/LV
(n=983) |
|
|---|---|---|
| Age (median, years) | 62 | 63 |
| Range | (25 to 80) | (22 to 82) |
| Gender | ||
| Male (n, %) | 542 (54) | 532 (54) |
| Female (n, %) | 461 (46) | 451 (46) |
| ECOG PS | ||
| 0 (n, %) | 849 (85) | 830 (85) |
| 1 (n, %) | 152 (15) | 147 (15) |
| Staging – Primary Tumor | ||
| PT1 (n, %) | 12 (1) | 6 (0.6) |
| PT2 (n, %) | 90 (9) | 92 (9) |
| PT3 (n, %) | 763 (76) | 746 (76) |
| PT4 (n, %) | 138 (14) | 139 (14) |
| Other (n, %) | 1 (0.1) | 0 (0) |
| Staging – Lymph Node | ||
| pN1 (n, %) | 695 (69) | 694 (71) |
| pN2 (n, %) | 305 (30) | 288 (29) |
| Other (n, %) | 4 (0.4) | 1 (0.1) |
All patients with normal renal function or mild renal impairment began treatment at the full starting dose of 1250 mg/m 2 orally twice daily. The starting dose was reduced in patients with moderate renal impairment (calculated creatinine clearance 30 to 50 mL/min) at baseline [see Dosage and Administration (2.4)] . Subsequently, for all patients, doses were adjusted when needed according to toxicity. Dose management for capecitabine included dose reductions, cycle delays and treatment interruptions (see Table 11).
| Capecitabine
N = 995 | 5-FU/LV
N = 974 |
|
|---|---|---|
| Median relative dose intensity (%) | 93 | 92 |
| Patients completing full course of treatment (%) | 83 | 87 |
| Patients with treatment interruption (%) | 15 | 5 |
| Patients with cycle delay (%) | 46 | 29 |
| Patients with dose reduction (%) | 42 | 44 |
| Patients with treatment interruption, cycle delay, or dose reduction (%) | 57 | 52 |
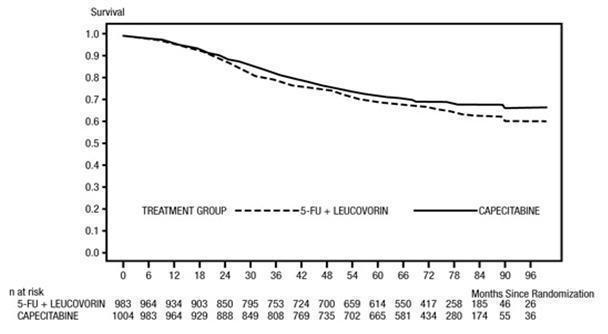
The median follow-up at the time of the analysis was 83 months (6.9 years). The hazard ratio for DFS for capecitabine compared to 5-FU/LV was 0.88 (95% C.I. 0.77 to 1.01) (see Table 12 and Figure 1). Because the upper 2-sided 95% confidence limit of hazard ratio was less than 1.20, capecitabine was non-inferior to 5-FU/LV. The choice of the non-inferiority margin of 1.20 corresponds to the retention of approximately 75% of the 5-FU/LV effect on DFS. The hazard ratio for capecitabine compared to 5-FU/LV with respect to overall survival was 0.86 (95% C.I. 0.74 to 1.01). The 5-year overall survival rates were 71.4% for capecitabine and 68.4% for 5-FU/LV (see Figure 2) .
| All Randomized Population | Capecitabine (n=1004) | 5-FU/LV
(n=983) |
|---|---|---|
| Median follow-up (months) | 83 | 83 |
| 5-year Disease-free Survival Rates (%) † | 59.1 | 54.6 |
| Hazard Ratio | 0.88 | |
| (Capecitabine/5-FU/LV)
| (0.77 to 1.01) | |
| (95% C.I. for Hazard Ratio) | ||
| p-value ‡ | p = 0.068 | |
|
| Figure 1 Kaplan-Meier Estimates of Disease-Free Survival (All Randomized Population)
*
|
Figure 2 Kaplan-Meier Estimates of Overall Survival (All Randomized Population)
14.2 Metastatic Colorectal Cancer
General
The recommended dose of capecitabine was determined in an open-label, randomized clinical study, exploring the efficacy and safety of continuous therapy with capecitabine (1331 mg/m 2/day in two divided doses, n=39), intermittent therapy with capecitabine (2510 mg/m 2/day in two divided doses, n=34), and intermittent therapy with capecitabine in combination with oral leucovorin (LV) (capecitabine 1657 mg/m 2/day in two divided doses, n=35; leucovorin 60 mg/day) in patients with advanced and/or metastatic colorectal carcinoma in the first-line metastatic setting. There was no apparent advantage in response rate to adding leucovorin to capecitabine; however, toxicity was increased. Capecitabine, 1250 mg/m 2 twice daily for 14 days followed by a 1-week rest, was selected for further clinical development based on the overall safety and efficacy profile of the three schedules studied.
Monotherapy
Data from two open-label, multicenter, randomized, controlled clinical trials involving 1207 patients support the use of capecitabine in the first-line treatment of patients with metastatic colorectal carcinoma. The two clinical studies were identical in design and were conducted in 120 centers in different countries. Study 1 was conducted in the US, Canada, Mexico, and Brazil; Study 2 was conducted in Europe, Israel, Australia, New Zealand, and Taiwan. Altogether, in both trials, 603 patients were randomized to treatment with capecitabine at a dose of 1250 mg/m 2 twice daily for 2 weeks followed by a 1-week rest period and given as 3-week cycles; 604 patients were randomized to treatment with 5-FU and leucovorin (20 mg/m 2 leucovorin IV followed by 425 mg/m 2 IV bolus 5-FU, on days 1 to 5, every 28 days).
In both trials, overall survival, time to progression and response rate (complete plus partial responses) were assessed. Responses were defined by the World Health Organization criteria and submitted to a blinded independent review committee (IRC). Differences in assessments between the investigator and IRC were reconciled by the sponsor, blinded to treatment arm, according to a specified algorithm. Survival was assessed based on a non-inferiority analysis.
The baseline demographics for capecitabine and 5-FU/LV patients are shown in Table 13.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| Capecitabine
(n=302) | 5-FU/LV
(n=303) | Capecitabine
(n=301) | 5-FU/LV
(n=301) |
|
| Age (median, years) | 64 | 63 | 64 | 64 |
| Range | (23 to 86) | (24 to 87) | (29 to 84) | (36 to 86) |
| Gender | ||||
| Male (%) | 181 (60) | 197 (65) | 172 (57) | 173 (57) |
| Female (%) | 121 (40) | 106 (35) | 129 (43) | 128 (43) |
| Karnofsky PS (median) | 90 | 90 | 90 | 90 |
| Range | (70 to 100) | (70 to 100) | (70 to 100) | (70 to 100) |
| Colon (%)
| 222 (74) | 232 (77) | 199 (66) | 196 (65) |
| Rectum (%) | 79 (26) | 70 (23) | 101 (34) | 105 (35) |
| Prior radiation therapy (%) | 52 (17) | 62 (21) | 42 (14) | 42 (14) |
| Prior adjuvant 5-FU (%) | 84 (28) | 110 (36) | 56 (19) | 41 (14) |
The efficacy endpoints for the two phase 3 trials are shown in Table 14 and Table 15.
| Capecitabine
(n=302) | 5-FU/LV
(n=303) |
|
|---|---|---|
| Overall Response Rate
(%, 95% C.I.) | 21 (16 to 26) | 11 (8 to 15) |
| ( p-value) | 0.0014 | |
| Time to Progression
(Median, days, 95% C.I.) | 128 (120 to 136) | 131 (105 to 153) |
| Hazard Ratio (capecitabine/5-FU/LV) | 0.99 | |
| 95% C.I. for Hazard Ratio | (0.84 to 1.17) | |
| Survival
(Median, days, 95% C.I.) |
380 (321 to 434) |
407 (366 to 446) |
| Hazard Ratio (capecitabine/5-FU/LV) | 1.00 | |
| 95% C.I. for Hazard Ratio | (0.84 to 1.18) | |
| Capecitabine
(n=301) | 5-FU/LV
(n=301) |
|
|---|---|---|
| Overall Response Rate
(%, 95% C.I.) | 21 (16-26) | 14 (10-18) |
| ( p-value) | 0.027 | |
| Time to Progression
(Median, days, 95% C.I.) | 137 (128-165) | 131 (102-156) |
| Hazard Ratio (Capecitabine/5-FU/LV) | 0.97 | |
| 95% C.I. for Hazard Ratio | (0.82-1.14) | |
| Survival
(Median, days, 95% C.I.) |
404 (367-452) |
369 (338-430) |
| Hazard Ratio (Capecitabine/5-FU/LV) | 0.92 | |
| 95% C.I. for Hazard Ratio | (0.78-1.09) | |
Figure 3 Kaplan-Meier Curve for Overall Survival of Pooled Data (Studies 1 and 2)

Capecitabine was superior to 5-FU/LV for objective response rate in Study 1 and Study 2. The similarity of capecitabine and 5-FU/LV in these studies was assessed by examining the potential difference between the two treatments. In order to assure that capecitabine has a clinically meaningful survival effect, statistical analyses were performed to determine the percent of the survival effect of 5-FU/LV that was retained by capecitabine. The estimate of the survival effect of 5-FU/LV was derived from a meta-analysis of ten randomized studies from the published literature comparing 5-FU to regimens of 5-FU/LV that were similar to the control arms used in these Studies 1 and 2. The method for comparing the treatments was to examine the worst case (95% confidence upper bound) for the difference between 5-FU/LV and capecitabine, and to show that loss of more than 50% of the 5-FU/LV survival effect was ruled out. It was demonstrated that the percent of the survival effect of 5-FU/LV maintained was at least 61% for Study 2 and 10% for Study 1. The pooled result is consistent with a retention of at least 50% of the effect of 5-FU/LV. It should be noted that these values for preserved effect are based on the upper bound of the 5-FU/LV vs capecitabine difference. These results do not exclude the possibility of true equivalence of capecitabine to 5-FU/LV (see Table 14, Table 15, and Figure 3).
14.3 Breast Cancer
Capecitabine has been evaluated in clinical trials in combination with docetaxel (Taxotere®) and as monotherapy.
In Combination With Docetaxel
The dose of capecitabine used in the phase 3 clinical trial in combination with docetaxel was based on the results of a phase 1 study, where a range of doses of docetaxel administered in 3-week cycles in combination with an intermittent regimen of capecitabine (14 days of treatment, followed by a 7-day rest period) were evaluated. The combination dose regimen was selected based on the tolerability profile of the 75 mg/m 2 administered in 3-week cycles of docetaxel in combination with 1250 mg/m 2 twice daily for 14 days of capecitabine administered in 3-week cycles. The approved dose of 100 mg/m 2 of docetaxel administered in 3-week cycles was the control arm of the phase 3 study.
Capecitabine in combination with docetaxel was assessed in an open-label, multicenter, randomized trial in 75 centers in Europe, North America, South America, Asia, and Australia. A total of 511 patients with metastatic breast cancer resistant to, or recurring during or after an anthracycline-containing therapy, or relapsing during or recurring within 2 years of completing an anthracycline-containing adjuvant therapy were enrolled. Two hundred and fifty-five (255) patients were randomized to receive capecitabine 1250 mg/m 2 twice daily for 14 days followed by 1 week without treatment and docetaxel 75 mg/m 2 as a 1-hour intravenous infusion administered in 3-week cycles. In the monotherapy arm, 256 patients received docetaxel 100 mg/m 2 as a 1-hour intravenous infusion administered in 3-week cycles. Patient demographics are provided in Table 16.
| Capecitabine + Docetaxel
(n=255) | Docetaxel
(n=256) |
|
|---|---|---|
|
||
| Age (median, years) | 52 | 51 |
| Karnofsky PS (median) | 90 | 90 |
| Site of Disease | ||
| Lymph nodes | 121 (47%) | 125 (49%) |
| Liver | 116 (45%) | 122 (48%) |
| Bone | 107 (42%) | 119 (46%) |
| Lung | 95 (37%) | 99 (39%) |
| Skin | 73 (29%) | 73 (29%) |
| Prior Chemotherapy | ||
| Anthracycline * | 255 (100%) | 256 (100%) |
| 5-FU | 196 (77%) | 189 (74%) |
| Paclitaxel | 25 (10%) | 22 (9%) |
| Resistance to an Anthracycline | ||
| No resistance | 19 (7%) | 19 (7%) |
| Progression on anthracycline therapy | 65 (26%) | 73 (29%) |
| Stable disease after 4 cycles of anthracycline therapy | 41 (16%) | 40 (16%) |
| Relapsed within 2 years of completion of anthracycline-adjuvant therapy | 78 (31%) | 74 (29%) |
| Experienced a brief response to anthracycline therapy, with subsequent progression while on therapy or within 12 months after last dose | 51 (20%) | 50 (20%) |
| No. of Prior Chemotherapy Regimens for Treatment of Metastatic Disease | ||
| 0 | 89 (35%) | 80 (31%) |
| 1 | 123 (48%) | 135 (53%) |
| 2 | 43 (17%) | 39 (15%) |
| 3 | 0 (0%) | 2 (1%) |
Capecitabine in combination with docetaxel resulted in statistically significant improvement in time to disease progression, overall survival and objective response rate compared to monotherapy with docetaxel as shown in Table 17, Figure 4, and Figure 5.
| Efficacy Parameter | Combination Therapy | Monotherapy | p-value | Hazard Ratio |
|---|---|---|---|---|
| Time to Disease Progression | ||||
| Median Days | 186 | 128 | 0.0001 | 0.643 |
| 95% C.I. | (165 to 198) | (105 to 136) | ||
| Overall Survival | ||||
| Median Days | 442 | 352 | 0.0126 | 0.775 |
| 95% C.I. | (375 to 497) | (298 to 387) | ||
| Response Rate* | 32% | 22% | 0.009 | NA † |
Figure 4 Kaplan-Meier Estimates for Time to Disease Progression Capecitabine and Docetaxel vs Docetaxel

Figure 5 Kaplan-Meier Estimates of Survival Capecitabine and Docetaxel vs Docetaxel

Monotherapy
The antitumor activity of capecitabine as a monotherapy was evaluated in an open-label single-arm trial conducted in 24 centers in the US and Canada. A total of 162 patients with stage IV breast cancer were enrolled. The primary endpoint was tumor response rate in patients with measurable disease, with response defined as a ≥50% decrease in sum of the products of the perpendicular diameters of bidimensionally measurable disease for at least 1 month. Capecitabine was administered at a dose of 1255 mg/m 2 twice daily for 2 weeks followed by a 1-week rest period and given as 3-week cycles. The baseline demographics and clinical characteristics for all patients (n=162) and those with measurable disease (n=135) are shown in Table 18. Resistance was defined as progressive disease while on treatment, with or without an initial response, or relapse within 6 months of completing treatment with an anthracycline-containing adjuvant chemotherapy regimen.
| Patients With Measurable Disease (n=135) |
All Patients (n=162) |
|
|---|---|---|
| Age (median, years) | 55 | 56 |
| Karnofsky PS | 90 | 90 |
| No. Disease Sites | ||
| 1 to 2 | 43 (32%) | 60 (37%) |
| 3 to 4 | 63 (46%) | 69 (43%) |
| >5 | 29 (22%) | 34 (21%) |
| Dominant Site of Disease | ||
| Visceral * | 101 (75%) | 110 (68%) |
| Soft Tissue | 30 (22%) | 35 (22%) |
| Bone | 4 (3%) | 17 (10%) |
| Prior Chemotherapy | ||
| Paclitaxel | 135 (100%) | 162 (100%) |
| Anthracycline † | 122 (90%) | 147 (91%) |
| 5-FU | 110 (81%) | 133 (82%)
|
| Resistance to Paclitaxel | 103 (76%) | 124 (77%) |
| Resistance to an Anthracycline † | 55 (41%) | 67 (41%) |
| Resistance to both Paclitaxel and an Anthracycline † | 43 (32%) | 51 (31%) |
Antitumor responses for patients with disease resistant to both paclitaxel and an anthracycline are shown in Table 19.
| Resistance to Both Paclitaxel and an Anthracycline
(n=43) |
|
|---|---|
| CR | 0 |
| PR * | 11 |
| CR + PR * | 11 |
| Response Rate * | 25.6% |
| (95% C.I.) | (13.5, 41.2) |
| Duration of Response, * | |
| Median in days † | 154 |
| (Range) | (63 to 233) |
For the subgroup of 43 patients who were doubly resistant, the median time to progression was 102 days and the median survival was 255 days. The objective response rate in this population was supported by a response rate of 18.5% (1 CR, 24 PRs) in the overall population of 135 patients with measurable disease, who were less resistant to chemotherapy (see Table 18). The median time to progression was 90 days and the median survival was 306 days.
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
150 mg
Color: Light peach
Engraving: 150 on one side, plain on the other
150 mg tablets are packaged in bottles of 60 (NDC 16714-467-01) individually packaged in a carton.
500 mg
Color: Peach
Engraving: 500 on one side, plain on the other
500 mg tablets are packaged in bottles of 120 (NDC 16714-468-01) individually packaged in a carton.
Storage and Handling
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]. KEEP TIGHTLY CLOSED.
Capecitabine tablets, USP are cytotoxic drug. Follow applicable special handling and disposal procedures. 1 Any unused product should be disposed of in accordance with local requirements, or drug take back programs.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Patient Information).
Diarrhea
Inform patients experiencing grade 2 diarrhea (an increase of 4 to 6 stools/day or nocturnal stools) or greater or experiencing severe bloody diarrhea with severe abdominal pain and fever to stop taking capecitabine. Advise patients on the use of antidiarrheal treatments (e.g., loperamide) to manage diarrhea [see Warnings and Precautions (5.2)].
Cardiotoxicity
Advise patients of the risk of cardiotoxicity and to immediately contact their healthcare provider or to go to an emergency room for new onset of chest pain, shortness of breath, dizziness, or lightheadedness [see Warnings and Precautions (5.3)].
Dihydropyrimidine Dehydrogenase Deficiency
Advise patients to notify their healthcare provider if they have a known DPD deficiency. Advise patients if they have complete or near complete absence of DPD activity they are at an increased risk of acute early-onset of toxicity and severe, life-threatening, or fatal adverse reactions caused by capecitabine (e.g., mucositis, diarrhea, neutropenia, and neurotoxicity) [see Warnings and Precautions (5.4)].
Dehydration and Renal Failure
Instruct patients experiencing grade 2 or higher dehydration (IV fluids indicated < 24 hours) to stop taking capecitabine immediately and to call their healthcare provider to correct the dehydration. Advise patients to not restart capecitabine until rehydrated and any precipitating causes have been corrected or controlled [see Warnings and Precautions (5.5)]. .
Important Administration Instructions
Advise patients to swallow capecitabine tablets whole with water within 30 minutes of a meal. Advise patients and caregivers not to crush or cut capecitabine tablets. Advise patients if they cannot swallow capecitabine tablets whole, to inform their healthcare provider [see Dosage and Administration (2.1)]. .
Hypersensitivity and Angioedema
Advise patients that capecitabine tablets may cause severe hypersensitivity reactions and angioedema. Advise patients who have known hypersensitivity to capecitabine or 5-fluorouracil to inform their healthcare provider [see Contraindications (4)]. Instruct patients who develop hypersensitivity reactions or mucocutaneous symptoms (e.g., urticaria, rash, erythema, pruritus, or swelling of the face, lips, tongue or throat which make it difficult to swallow or breathe) to stop taking capecitabine tablets and immediately contact their healthcare provider or to go to an emergency room. [see Adverse Reactions (6)].
Nausea
Instruct patients experiencing grade 2 nausea (food intake significantly decreased but able to eat intermittently) or greater to stop taking capecitabine immediately and to contact their healthcare provider for management of nausea [see Adverse Reactions (6.1)]. .
Vomiting
Instruct patients experiencing grade 2 vomiting (2 to 5 episodes in a 24-hour period) or greater to stop taking capecitabine immediately and to contact their healthcare provider for management of vomiting [see Adverse Reactions (6.1)]. .
Hand-and-Foot Syndrome
Instruct patients experiencing grade 2 hand-and-foot syndrome (painful erythema and swelling of the hands and/or feet and/or discomfort affecting the patients’ activities of daily living) or greater to stop taking capecitabine immediately and to contact their healthcare provider. Inform patients that initiation of symptomatic treatment is recommended, and hand-and-foot syndrome can lead to loss of fingerprints which could impact personal identification [see Adverse Reactions (6.1)]. .
Stomatitis
Inform experiencing grade 2 stomatitis (painful erythema, edema or ulcers of the mouth or tongue, but able to eat) or greater to stop taking capecitabine immediately and to contact their healthcare provider [see Adverse Reactions (6.1)]. .
Fever and Neutropenia
Inform patients who develop a fever of 100.5°F or greater or other evidence of potential infection to contact their healthcare provider [see Adverse Reactions (6.1)]. .
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception during treatment with capecitabine tablets and for 6 months after the last dose. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.6), Use in Specific Populations (8.1 and 8.3)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with capecitabine tablets and for 3 months after the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise females not to breastfeed during treatment with capecitabine tablets and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
For full Taxotere prescribing information, please refer to Taxotere Package Insert.
Xeloda is a registered trademark of Hoffmann-La Roche Inc.
All trade names drug products are the property of their respective owners.
Manufactured For:
Northstar Rx LLC
Memphis, TN 38141
Manufactured By:
Intas Pharmaceuticals Limited,
Ahmedabad – 380 054, India.
10 1774 2 6012366
Revised 10/2021
SPL UNCLASSIFIED SECTION
INGREDIENTS AND APPEARANCE
| CAPECITABINE
capecitabine tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| CAPECITABINE
capecitabine tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - NORTHSTAR RXLLC (830546433) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Intas Pharmaceuticals Ltd | 725927649 | manufacture(16714-468, 16714-467) , analysis(16714-468, 16714-467) | |
