Search by Drug Name or NDC
NDC 17478-0715-11 Brimonidine 2 mg/mL Details
Brimonidine 2 mg/mL
Brimonidine is a OPHTHALMIC SOLUTION/ DROPS in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Akorn. The primary component is BRIMONIDINE TARTRATE.
MedlinePlus Drug Summary
Ophthalmic brimonidine is used to lower pressure in the eyes in patients who have glaucoma (high pressure in the eyes that may damage nerves and cause vision loss) and ocular hypertension (pressure in the eyes that is higher than normal but not high enough to cause vision loss). Brimonidine is in a class of drugs called alpha adrenergic agonists. Brimonidine works by decreasing the amount of fluid in the eyes.
Related Packages: 17478-0715-11Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Brimonidine Ophthalmic
Product Information
| NDC | 17478-0715 |
|---|---|
| Product ID | 17478-715_edc8b30f-509f-44c1-b498-8cf9672aba66 |
| Associated GPIs | 86602020102010 |
| GCN Sequence Number | 027882 |
| GCN Sequence Number Description | brimonidine tartrate DROPS 0.2 % OPHTHALMIC |
| HIC3 | Q6G |
| HIC3 Description | MIOTICS AND OTHER INTRAOCULAR PRESSURE REDUCERS |
| GCN | 36281 |
| HICL Sequence Number | 011786 |
| HICL Sequence Number Description | BRIMONIDINE TARTRATE |
| Brand/Generic | Generic |
| Proprietary Name | Brimonidine |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Brimonidine Tartrate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SOLUTION/ DROPS |
| Route | OPHTHALMIC |
| Active Ingredient Strength | 2 |
| Active Ingredient Units | mg/mL |
| Substance Name | BRIMONIDINE TARTRATE |
| Labeler Name | Akorn |
| Pharmaceutical Class | Adrenergic alpha-Agonists [MoA], alpha-Adrenergic Agonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA076439 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
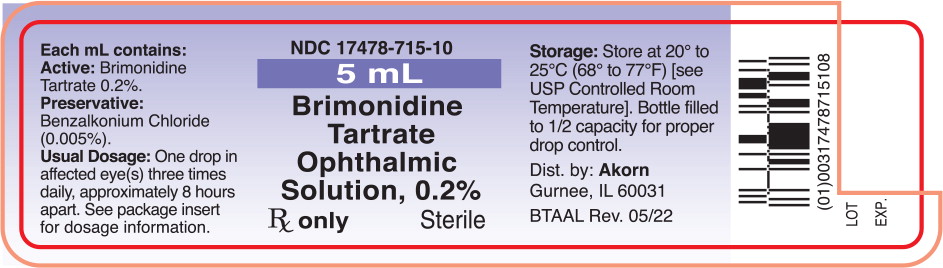

NDC 17478-0715-11 (17478071511)
| NDC Package Code | 17478-715-11 |
|---|---|
| Billing NDC | 17478071511 |
| Package | 1 BOTTLE, DROPPER in 1 CARTON (17478-715-11) / 10 mL in 1 BOTTLE, DROPPER |
| Marketing Start Date | 2006-09-07 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.59121 |
| Pricing Unit | ML |
| Effective Date | 2022-11-23 |
| NDC Description | BRIMONIDINE 0.2% EYE DROP |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1, 5 |
| Classification for Rate Setting | G |
| As of Date | 2022-11-23 |
Standard Product Labeling (SPL)/Prescribing Information SPL 1ce6d79a-32c1-44dc-b50c-ada8848f39c3 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
BRIMONIDINE TARTRATE ophthalmic solution, for topical ophthalmic use
Initial U.S. Approval: 1996
INDICATIONS AND USAGE
Brimonidine tartrate ophthalmic solution 0.2% is an alpha adrenergic agonist indicated for lowering intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. (1)
DOSAGE AND ADMINISTRATION
One drop in the affected eye(s), three times daily, approximately 8 hours apart. (2)
DOSAGE FORMS AND STRENGTHS
Solution containing 2 mg/mL brimonidine tartrate. (3)
CONTRAINDICATIONS
Neonates and infants (under the age of 2 years). (4.1)
WARNINGS AND PRECAUTIONS
Potentiation of vascular insufficiency. (5.1)
ADVERSE REACTIONS
Most common adverse reactions occurring in approximately 10% to 30% of patients receiving brimonidine ophthalmic solution 0.2% included oral dryness, ocular hyperemia, burning and stinging, headache, blurring, foreign body sensation, fatigue/drowsiness, conjunctival follicles, ocular allergic reactions, and ocular pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Akorn Operating Company LLC at 1-800-932-5676 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Antihypertensives/cardiac glycosides may lower blood pressure. (7.1)
- Use with CNS depressants may result in an additive or potentiating effect. (7.2)
- Tricyclic antidepressants may potentially blunt the hypotensive effect of systemic clonidine. (7.3)
- Monoamine oxidase inhibitors may result in increased hypotension. (7.4)
USE IN SPECIFIC POPULATIONS
Use with caution in children ≥ 2 years of age. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Neonates and Infants (under the age of 2 years)
4.2 Hypersensitivity Reactions
5 WARNINGS AND PRECAUTIONS
5.1 Potentiation of Vascular Insufficiency
5.2 Severe Cardiovascular Disease
5.3 Contamination of Topical Ophthalmic Products After Use
5.4 Use with Contact Lenses
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antihypertensives/Cardiac Glycosides
7.2 CNS Depressants
7.3 Tricyclic Antidepressants
7.4 Monoamine Oxidase Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Special Populations
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
Brimonidine tartrate ophthalmic solution 0.2% is indicated for lowering intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
The IOP lowering efficacy of brimonidine tartrate ophthalmic solution diminishes over time in some patients. This loss of effect appears with a variable time of onset in each patient and should be closely monitored.
2 DOSAGE AND ADMINISTRATION
The recommended dose is one drop of brimonidine tartrate ophthalmic solution 0.2% in the affected eye(s) three times daily, approximately 8 hours apart.
Brimonidine tartrate ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic product is to be used, the different products should be instilled at least 5 minutes apart.
4 CONTRAINDICATIONS
4.1 Neonates and Infants (under the age of 2 years)
Brimonidine tartrate ophthalmic solution is contraindicated in neonates and infants (under the age of 2 years) [see Use in Specific Populations (8.4)].
5 WARNINGS AND PRECAUTIONS
5.1 Potentiation of Vascular Insufficiency
Brimonidine tartrate ophthalmic solution may potentiate syndromes associated with vascular insufficiency.
Brimonidine tartrate ophthalmic solution should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud's phenomenon, orthostatic hypotension, or thromboangiitis obliterans.
5.2 Severe Cardiovascular Disease
Although brimonidine tartrate ophthalmic solution had minimal effect on the blood pressure of patients in clinical studies, caution should be exercised in treating patients with severe cardiovascular disease.
5.3 Contamination of Topical Ophthalmic Products After Use
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface [see Patient Counseling Information (17)].
5.4 Use with Contact Lenses
The preservative in brimonidine tartrate ophthalmic solution, benzalkonium chloride, may be absorbed by soft contact lenses. Patients wearing soft contact lenses should be instructed to wait at least 15 minutes after instilling brimonidine tartrate ophthalmic solution to insert soft contact lenses.
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Potentiation of Vascular Insufficiency [see Warnings and Precautions (5.1)]
- Severe Cardiovascular Disease [see Warnings and Precautions (5.2)]
- Contamination of Topical Ophthalmic Products after Use [see Warnings and Precautions (5.3)]
- Neonates and Infants (under the age of 2 years) [see Contraindications (4.1)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
- Adverse reactions occurring in approximately 10% to 30% of the subjects (in descending order): oral dryness, ocular hyperemia, burning and stinging, headache, blurring, foreign body sensation, fatigue/drowsiness, conjunctival follicles, ocular allergic reactions, and ocular pruritus.
- Adverse reactions occurring in approximately 3% to 9% of the subjects (in descending order): corneal staining/erosion, photophobia, eyelid erythema, ocular ache/pain, ocular dryness, tearing, upper respiratory symptoms, eyelid edema, conjunctival edema, dizziness, blepharitis, ocular irritation, gastrointestinal symptoms, asthenia, conjunctival blanching, abnormal vision and muscular pain.
- Adverse reactions reported < 3% of the patients: lid crusting, conjunctival hemorrhage, abnormal taste, insomnia, conjunctival discharge, depression, hypertension, anxiety, palpitations/arrhythmias, nasal dryness and syncope.
6.2 Postmarketing Experience
The following reactions have been identified during postmarketing use of brimonidine tartrate ophthalmic solutions in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to brimonidine tartrate ophthalmic solutions, or a combination of these factors, include:
- Bradycardia; conjunctivitis; hypersensitivity; hypotension; iritis; keratoconjunctivitis sicca; lacrimation increased; miosis; nausea; skin reactions (including erythema, eyelid pruritus, rash, and vasodilation); and tachycardia.
- Apnea, bradycardia, coma, hypotension, hypothermia, hypotonia, lethargy, pallor, respiratory depression, and somnolence in infants receiving brimonidine tartrate ophthalmic solutions.
7 DRUG INTERACTIONS
7.1 Antihypertensives/Cardiac Glycosides
Because brimonidine tartrate ophthalmic solution may reduce blood pressure, caution in using drugs such as antihypertensives and/or cardiac glycosides with brimonidine tartrate ophthalmic solution is advised.
7.2 CNS Depressants
Although specific drug interaction studies have not been conducted with brimonidine tartrate ophthalmic solution, the possibility of an additive or potentiating effect with CNS depressants (alcohol, barbiturates, opiates, sedatives, or anesthetics) should be considered.
7.3 Tricyclic Antidepressants
Tricyclic antidepressants have been reported to blunt the hypotensive effect of systemic clonidine. It is not known whether the concurrent use of these agents with brimonidine tartrate ophthalmic solution in humans can lead to resulting interference with the IOP lowering effect. Caution is advised in patients taking tricyclic antidepressants which can affect the metabolism and uptake of circulating amines.
7.4 Monoamine Oxidase Inhibitors
Monoamine oxidase (MAO) inhibitors may theoretically interfere with the metabolism of brimonidine and potentially result in an increased systemic side-effect such as hypotension. Caution is advised in patients taking MAO inhibitors which can affect the metabolism and uptake of circulating amines.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B: Teratogenicity studies have been performed in animals.
Brimonidine tartrate was not teratogenic when given orally during gestation days 6 through 15 in rats and days 6 through 18 in rabbits. The highest doses of brimonidine tartrate in rats (2.5 mg/kg/day) and rabbits (5 mg/kg/day) achieved AUC exposure values 375-fold higher or 19-fold higher, respectively, than similar values estimated in humans treated with brimonidine tartrate ophthalmic solution 0.2%, one drop in one eye, twice daily.
There are no adequate and well-controlled studies in pregnant women; however, in animal studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent. Because animal reproduction studies are not always predictive of human response, brimonidine tartrate ophthalmic solution should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether brimonidine tartrate is excreted in human milk, although in animal studies, brimonidine tartrate has been shown to be excreted in breast milk. Because of the potential for serious adverse reactions from brimonidine tartrate ophthalmic solution in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Brimonidine tartrate ophthalmic solution is contraindicated in children under the age of 2 years [see Contraindications (4.1)]. During postmarketing surveillance, apnea, bradycardia, coma, hypotension, hypothermia, hypotonia, lethargy, pallor, respiratory depression, and somnolence have been reported in infants receiving brimonidine. The safety and effectiveness of brimonidine tartrate have not been studied in children below the age of 2 years.
In a well-controlled clinical study conducted in pediatric glaucoma patients (ages 2 to 7 years) the most commonly observed adverse reactions with brimonidine tartrate ophthalmic solution 0.2% dosed three times daily were somnolence (50% to 83% in patients ages 2 to 6 years) and decreased alertness. In pediatric patients 7 years of age (greater than 20 kg), somnolence appears to occur less frequently (25%). Approximately 16% of patients on brimonidine tartrate ophthalmic solution 0.2% discontinued from the study due to somnolence.
8.5 Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
8.6 Special Populations
Brimonidine tartrate ophthalmic solution has not been studied in patients with hepatic impairment.
Brimonidine tartrate ophthalmic solution has not been studied in patients with renal impairment. The effect of dialysis on brimonidine pharmacokinetics in patients with renal failure is not known.
10 OVERDOSAGE
Very limited information exists on accidental ingestion of brimonidine in adults; the only adverse reaction reported to date has been hypotension. Symptoms of brimonidine overdose have been reported in neonates, infants, and children receiving brimonidine tartrate as part of medical treatment of congenital glaucoma or by accidental oral ingestion [see Use In Specific Populations (8.4)]. Treatment of an oral overdose includes supportive and symptomatic therapy; a patent airway should be maintained.
11 DESCRIPTION
Brimonidine tartrate ophthalmic solution 0.2%, sterile, is a relatively selective alpha-2 adrenergic receptor agonist (topical intraocular pressure lowering agent).
The structural formula of brimonidine tartrate is:
5-Bromo-6-(2-imidazolidinylideneamino) quinoxaline L-tartrate; MW= 442.24
In solution, brimonidine tartrate ophthalmic solution 0.2% has a clear, greenish-yellow color. It has an osmolality of 280 to 330 mOsml/kg and a pH of 5.6 to 6.6.
Each mL of brimonidine tartrate ophthalmic solution contains the active ingredient brimonidine tartrate 0.2% (2 mg/mL) with the inactive ingredients benzalkonium chloride 0.005% (0.05 mg/mL) as a preservative; citric acid; polyvinyl alcohol; sodium chloride; sodium citrate; and water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Brimonidine tartrate ophthalmic solution 0.2% is a relatively selective alpha-2 adrenergic receptor agonist with a peak ocular hypotensive effect occurring at two hours post-dosing.
Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and increasing uveoscleral outflow.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No compound-related carcinogenic effects were observed in either mice or rats following a 21-month and 24-month study, respectively. In these studies, dietary administration of brimonidine tartrate at doses up to 2.5 mg/kg/day in mice and 1.0 mg/kg/day in rats achieved ~77 and 118 times, respectively, the plasma Cmax drug concentration estimated in humans treated with one drop brimonidine tartrate ophthalmic solution 0.2% into both eyes 2 times per day.
Brimonidine tartrate was not mutagenic or clastogenic in a series of in vitro and in vivo studies including the Ames bacterial reversion test, chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells, and three in vivo studies in CD-1 mice: a host-mediated assay, cytogenetic study, and dominant lethal assay.
A reproduction and fertility study in rats with brimonidine tartrate demonstrated no adverse effect on male or female fertility at oral doses up to 1 mg/kg, estimated as approximately 200 times the systemic exposure (AUC) following the maximum recommended human ophthalmic dose of brimonidine tartrate ophthalmic solution 0.5%.
14 CLINICAL STUDIES
Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Brimonidine tartrate has the action of lowering intraocular pressure with minimal effect on cardiovascular and pulmonary parameters.
In comparative clinical studies with timolol 0.5%, lasting up to one year, the IOP lowering effect of brimonidine tartrate ophthalmic solution was approximately 4 to 6 mm Hg compared with approximately 6 mm Hg for timolol. In these studies, both patient groups were dosed BID; however, due to the duration of action of brimonidine tartrate ophthalmic solution, it is recommended that brimonidine tartrate ophthalmic solution be dosed TID. Eight percent of subjects were discontinued from studies due to inadequately controlled intraocular pressure, which in 30% of these patients occurred during the first month of therapy. Approximately 20% were discontinued due to adverse experiences.
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Handling the Container
Instruct patients that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions [see Warnings and Precautions (5.3)]. Always replace the cap after using. If solution changes color or becomes cloudy, do not use. Do not use the product after the expiration date marked on the bottle.
When to Seek Physician Advice
Advise patients that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container.
Use with Contact Lenses
Advise patients that contact lenses should be removed prior to instillation of brimonidine tartrate ophthalmic solution and may be reinserted 15 minutes following its administration.
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
Potential for Decreased Mental Alertness
As with other similar medications, brimonidine tartrate ophthalmic solution may cause fatigue and/or drowsiness in some patients. Caution patients who engage in hazardous activities of the potential for a decrease in mental alertness.
AKORN
Distributed by:
Akorn Operating Company LLC
Gurnee, IL 60031
BT00N Rev. 05/22
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| BRIMONIDINE
brimonidine tartrate solution/ drops |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Akorn (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696832 | MANUFACTURE(17478-715) , ANALYSIS(17478-715) , STERILIZE(17478-715) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696790 | PACK(17478-715) , LABEL(17478-715) | |
