Search by Drug Name or NDC
NDC 23155-0732-10 Nadolol 80 mg/1 Details
Nadolol 80 mg/1
Nadolol is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.. The primary component is NADOLOL.
MedlinePlus Drug Summary
Nadolol is used alone or in combination with other medications to treat high blood pressure. It is also used to prevent angina (chest pain). Nadolol is in a class of medications called beta blockers. It works by relaxing blood vessels and slowing heart rate to improve blood flow and decrease blood pressure. High blood pressure is a common condition and when not treated, can cause damage to the brain, heart, blood vessels, kidneys, and other parts of the body. Damage to these organs may cause heart disease, a heart attack, heart failure, stroke, kidney failure, loss of vision, and other problems. In addition to taking medication, making lifestyle changes will also help to control your blood pressure. These changes include eating a diet that is low in fat and salt, maintaining a healthy weight, exercising at least 30 minutes most days, not smoking, and using alcohol in moderation.
Related Packages: 23155-0732-10Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Nadolol
Product Information
| NDC | 23155-0732 |
|---|---|
| Product ID | 23155-732_7ce5fe0a-cf3d-4ef6-892c-b737f87c33e1 |
| Associated GPIs | 33100010000310 |
| GCN Sequence Number | 005137 |
| GCN Sequence Number Description | nadolol TABLET 80 MG ORAL |
| HIC3 | J7C |
| HIC3 Description | BETA-ADRENERGIC BLOCKING AGENTS |
| GCN | 20653 |
| HICL Sequence Number | 002103 |
| HICL Sequence Number Description | NADOLOL |
| Brand/Generic | Generic |
| Proprietary Name | Nadolol |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Nadolol |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 80 |
| Active Ingredient Units | mg/1 |
| Substance Name | NADOLOL |
| Labeler Name | Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc. |
| Pharmaceutical Class | Adrenergic beta-Antagonists [MoA], beta-Adrenergic Blocker [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA074255 |
| Listing Certified Through | 2022-12-31 |
Package
Package Images



NDC 23155-0732-10 (23155073210)
| NDC Package Code | 23155-732-10 |
|---|---|
| Billing NDC | 23155073210 |
| Package | 1000 TABLET in 1 BOTTLE (23155-732-10) |
| Marketing Start Date | 2021-02-26 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL fd76fabd-ac3e-4413-8383-c550d8006939 Details
DESCRIPTION
Nadolol Tablets USP are a synthetic nonselective beta-adrenergic receptor blocking agent designated chemically as 1-(tert-butylamino)-3-[(5,6,7,8-tetrahydro-cis-6,7-dihydroxy-1-naphthyl)oxy]-2-propanol. The structural formula is:
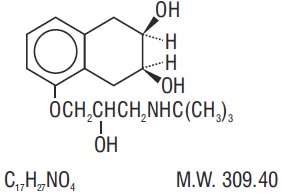
Nadolol, USP is a white to off-white, practically odorless, crystalline powder. It is freely soluble in alcohol and in methanol, soluble in water at pH 2, and slightly soluble in chloroform.
Nadolol Tablets USP are available for oral administration as 20 mg, 40 mg, and 80 mg tablets and contain the following inactive ingredients: citric acid anhydrous, corn starch, magnesium stearate, microcrystalline cellulose and povidone. In addition, the 80 mg tablets contain sodium starch glycolate.
CLINICAL PHARMACOLOGY
Nadolol is a nonselective beta-adrenergic receptor blocking agent. Clinical pharmacology studies have demonstrated beta-blocking activity by showing (1) reduction in heart rate and cardiac output at rest and on exercise, (2) reduction of systolic and diastolic blood pressure at rest and on exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia.
Nadolol specifically competes with beta-adrenergic receptor agonists for available beta-receptor sites; it inhibits both the beta1 receptors located chiefly in cardiac muscle and the beta2 receptors located chiefly in the bronchial and vascular musculature, inhibiting the chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation proportionately. Nadolol has no intrinsic sympathomimetic activity and, unlike some other beta-adrenergic blocking agents, nadolol has little direct myocardial depressant activity and does not have an anesthetic-like membrane-stabilizing action. Animal and human studies show that nadolol slows the sinus rate and depresses AV conduction. In dogs, only minimal amounts of nadolol were detected in the brain relative to amounts in blood and other organs and tissues. Nadolol has low lipophilicity as determined by octanol/ water partition coefficient, a characteristic of certain beta-blocking agents that has been correlated with the limited extent to which these agents cross the blood-brain barrier, their low concentration in the brain, and low incidence of CNS-related side effects.
In controlled clinical studies, nadolol at doses of 40 to 320 mg/day has been shown to decrease both standing and supine blood pressure, the effect persisting for approximately 24 hours after dosing.
The mechanism of the antihypertensive effects of beta-adrenergic receptor blocking agents has not been established; however factors that may be involved include (1) competitive antagonism of catecholamines at peripheral (non-CNS) adrenergic neuron sites (especially cardiac) leading to decreased cardiac output, (2) a central effect leading to reduced tonic-sympathetic nerve outflow to the periphery, and (3) suppression of renin secretion by blockade of the beta-adrenergic receptors responsible for renin release from the kidneys.
While cardiac output and arterial pressure are reduced by nadolol therapy, renal hemodynamics are stable, with preservation of renal blood flow and glomerular filtration rate.
By blocking catecholamine-induced increases in heart rate, velocity and extent of myocardial contraction, and blood pressure, nadolol generally reduces the oxygen requirements of the heart at any given level of effort, making it useful for many patients in the long-term management of angina pectoris. On the other hand, nadolol can increase oxygen requirements by increasing left ventricular fiber length and end diastolic pressure, particularly in patients with heart failure.
Although beta-adrenergic receptor blockade is useful in treatment of angina and hypertension, there are also situations in which sympathetic stimulation is vital. For example, in patients with severely damaged hearts, adequate ventricular function may depend on sympathetic drive. Beta-adrenergic blockade may worsen AV block by preventing the necessary facilitating effects of sympathetic activity on conduction. Beta2-adrenergic blockade results in passive bronchial constriction by interfering with endogenous adrenergic bronchodilator activity in patients subject to bronchospasm and may also interfere with exogenous bronchodilators in such patients.
Absorption of nadolol after oral dosing is variable, averaging about 30 percent. Peak serum concentrations of nadolol usually occur in three to four hours after oral administration and the presence of food in the gastrointestinal tract does not affect the rate or extent of nadolol absorption. Approximately 30 percent of the nadolol present in serum is reversibly bound to plasma protein.
Unlike many other beta-adrenergic blocking agents, nadolol is not metabolized by the liver and is excreted unchanged, principally by the kidneys.
The half-life of therapeutic doses of nadolol is about 20 to 24 hours, permitting once-daily dosage. Because nadolol is excreted predominantly in the urine, its half-life increases in renal failure (see PRECAUTIONS and DOSAGE AND ADMINISTRATION). Steady-state serum concentrations of nadolol are attained in six to nine days with once-daily dosage in persons with normal renal function. Because of variable absorption and different individual responsiveness, the proper dosage must be determined by titration.
Exacerbation of angina and, in some cases, myocardial infarction and ventricular dysrhythmias have been reported after abrupt discontinuation of therapy with beta-adrenergic blocking agents in patients with coronary artery disease. Abrupt withdrawal of these agents in patients without coronary artery disease has resulted in transient symptoms, including tremulousness, sweating, palpitation, headache, and malaise. Several mechanisms have been proposed to explain these phenomena, among them increased sensitivity to catecholamines because of increased numbers of beta receptors.
INDICATIONS AND USAGE
Angina Pectoris
Nadolol Tablets USP are indicated for the long-term management of patients with angina pectoris.
Hypertension
Nadolol Tablets USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Nadolol Tablets USP.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Nadolol Tablets USP may be used alone or in combination with other antihypertensive agents, especially thiazide-type diuretics.
CONTRAINDICATIONS
Nadolol is contraindicated in bronchial asthma, sinus bradycardia and greater than first degree conduction block, cardiogenic shock, and overt cardiac failure (see WARNINGS).
WARNINGS
Cardiac Failure
Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta-blockade may precipitate more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, if necessary, they can be used with caution in patients with a history of failure who are well-compensated, usually with digitalis and diuretics. Beta-adrenergic blocking agents do not abolish the inotropic action of digitalis on heart muscle.
IN PATIENTS WITHOUT A HISTORY OF HEART FAILURE, continued use of beta-blockers can, in some cases, lead to cardiac failure. Therefore, at the first sign or symptom of heart failure, the patient should be digitalized and/or treated with diuretics, and the response observed closely, or nadolol should be discontinued (gradually, if possible).
|
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered nadolol, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of one to two weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, nadolol administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue nadolol therapy abruptly even in patients treated only for hypertension. |
Nonallergic Bronchospasm (e.g., chronic bronchitis, emphysema)
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD IN GENERAL NOT RECEIVE BETA-BLOCKERS. Nadolol should be administered with caution since it may block bronchodilation produced by endogenous or exogenous catecholamine stimulation of beta2 receptors.
Major Surgery
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia
Beta-adrenergic blockade may prevent the appearance of premonitory signs and symptoms (e.g., tachycardia and blood pressure changes) of acute hypoglycemia. This is especially important with labile diabetics. Beta-blockade also reduces the release of insulin in response to hyperglycemia; therefore, it may be necessary to adjust the dose of antidiabetic drugs.
PRECAUTIONS
Impaired Renal Function
Nadolol should be used with caution in patients with impaired renal function (see DOSAGE AND ADMINISTRATION).
Information for Patients
Patients, especially those with evidence of coronary artery insufficiency, should be warned against interruption or discontinuation of nadolol therapy without the physician's advice. Although cardiac failure rarely occurs in properly selected patients, patients being treated with beta-adrenergic blocking agents should be advised to consult the physician at the first sign or symptom of impending failure. The patient should also be advised of a proper course in the event of an inadvertently missed dose.
Drug Interactions
When administered concurrently, the following drugs may interact with beta-adrenergic receptor blocking agents:
Anesthetics, General
Exaggeration of the hypotension induced by general anesthetics (see WARNINGS, Major Surgery).
Antidiabetic Drugs (oral agents and insulin)
Hypoglycemia or hyperglycemia; adjust dosage of antidiabetic drug accordingly (see WARNINGS, Diabetes and Hypoglycemia).
Catecholamine-depleting Drugs (e.g., reserpine)
Additive effect; monitor closely for evidence of hypotension and/or excessive bradycardia (e.g., vertigo, syncope, postural hypotension).
Digitalis Glycosides
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Response to Treatment for Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In chronic oral toxicologic studies (one to two years) in mice, rats, and dogs, nadolol did not produce any significant toxic effects. In two year oral carcinogenic studies in rats and mice, nadolol did not produce any neoplastic, preneoplastic, or non-neoplastic pathologic lesions. In fertility and general reproductive performance studies in rats, nadolol caused no adverse effects.
Pregnancy
Teratogenic Effects
Pregnancy Category C
In animal reproduction studies with nadolol, evidence of embryo- and fetotoxicity was found in rabbits, but not in rats or hamsters, at doses 5 to 10 times greater (on a mg/kg basis) than the maximum indicated human dose. No teratogenic potential was observed in any of these species.
There are no adequate and well-controlled studies in pregnant women. Nadolol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Neonates whose mothers are receiving nadolol at parturition have exhibited bradycardia, hypoglycemia, and associated symptoms.
ADVERSE REACTIONS
Most adverse effects have been mild and transient and have rarely required withdrawal of therapy.
Cardiovascular
Bradycardia with heart rates of less than 60 beats per minute occurs commonly, and heart rates below 40 beats per minute and/ or symptomatic bradycardia were seen in about 2 of 100 patients. Symptoms of peripheral vascular insufficiency, usually of the Raynaud type, have occurred in approximately 2 of 100 patients. Cardiac failure, hypotension, and rhythm/conduction disturbances have each occurred in about 1 of 100 patients. Single instances of first degree and third degree heart block have been reported; intensification of AV block is a known effect of beta-blockers (see also CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS).
Central Nervous System
Dizziness or fatigue has been reported in approximately 2 of 100 patients; paresthesias, sedation, and change in behavior have each been reported in approximately 6 of 1000 patients.
Respiratory
Bronchospasm has been reported in approximately 1 of 1000 patients (see CONTRAINDICATIONS and WARNINGS).
Gastrointestinal
Nausea, diarrhea, abdominal discomfort, constipation, vomiting, indigestion, anorexia, bloating, and flatulence have been reported in 1 to 5 of 1000 patients.
Miscellaneous
Each of the following has been reported in 1 to 5 of 1000 patients: rash; pruritus; headache; dry mouth, eyes, or skin; impotence or decreased libido; facial swelling; weight gain; slurred speech; cough; nasal stuffiness; sweating; tinnitus; blurred vision. Reversible alopecia has been reported infrequently.
The following adverse reactions have been reported in patients taking nadolol and/or other beta-adrenergic blocking agents, but no causal relationship to nadolol has been established.
Central Nervous System
Reversible mental depression progressing to catatonia; visual disturbances; hallucinations; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability with slightly clouded sensorium, and decreased performance on neuropsychometrics.
OVERDOSAGE
Nadolol can be removed from the general circulation by hemodialysis.
In addition to gastric lavage, the following measures should be employed, as appropriate. In determining the duration of corrective therapy, note must be taken of the long duration of the effect of nadolol.
Excessive Bradycardia
Administer atropine (0.25 to 1 mg). If there is no response to vagal blockade, administer isoproterenol cautiously.
Cardiac Failure
Administer a digitalis glycoside and diuretic. It has been reported that glucagon may also be useful in this situation.
DOSAGE AND ADMINISTRATION
DOSAGE MUST BE INDIVIDUALIZED. NADOLOL TABLETS MAY BE ADMINISTERED WITHOUT REGARD TO MEALS.
Angina Pectoris
The usual initial dose is 40 mg nadolol tablets once daily. Dosage may be gradually increased in 40 to 80 mg increments at 3 to 7 day intervals until optimum clinical response is obtained or there is pronounced slowing of the heart rate. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 160 or 240 mg administered once daily may be needed.
The usefulness and safety in angina pectoris of dosage exceeding 240 mg per day have not been established. If treatment is to be discontinued, reduce the dosage gradually over a period of one to two weeks (see WARNINGS).
Hypertension
The usual initial dose is 40 mg nadolol tablets once daily, whether it is used alone or in addition to diuretic therapy. Dosage may be gradually increased in 40 to 80 mg increments until optimum blood pressure reduction is achieved. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 240 or 320 mg administered once daily may be needed.
Dosage Adjustment in Renal Failure
Absorbed nadolol is excreted principally by the kidneys and, although nonrenal elimination does occur, dosage adjustments are necessary in patients with renal impairment. The following dose intervals are recommended:
|
Creatinine Clearance (mL/min/1.73m2) |
Dosage Interval (hours) |
|
> 50 |
24 |
|
31 to 50 |
24 to 36 |
|
10 to 30 |
24 to 48 |
|
< 10 |
40 to 60 |
HOW SUPPLIED
Nadolol Tablets USP, 20 mg are available as white round tablets debossed "730" on one side with break line and plain on other side, containing 20 mg of nadolol, USP packaged in bottles of 90 tablets (NDC 23155-730-09), 100 tablets (NDC 23155-730-01) and 1000 tablets (NDC 23155-730-10).
Nadolol Tablets USP, 40 mg are available as white round tablets debossed "731" on one side with break line and plain on other side, containing 40 mg of nadolol, USP packaged in bottles of 90 tablets (NDC 23155-731-09), 100 tablets (NDC 23155-731-01) and 1000 tablets (NDC 23155-731-10).
Nadolol Tablets USP, 80 mg are available as white round tablets debossed "732" on one side with break line and plain on other side, containing 80 mg of nadolol, USP packaged in bottles of 90 tablets (NDC 23155-732-09), 100 tablets (NDC 23155-732-01) and 1000 tablets (NDC 23155-732-10).
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured by:
RA CHEM PHARMA LIMITED
Hyderabad - 500 076, INDIA.
Distributed by:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 12/2019
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Nadolol Tablets USP, 20 mg
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Nadolol Tablets USP, 40 mg
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Nadolol Tablets USP, 80 mg
INGREDIENTS AND APPEARANCE
| NADOLOL
nadolol tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| NADOLOL
nadolol tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| NADOLOL
nadolol tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc. (780779901) |
| Registrant - Heritage Pharma Labs Inc. d/b/a Avet Pharmaceuticals Labs Inc. (189630168) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| RA CHEM PHARMA LIMITED | 677637710 | manufacture(23155-730, 23155-731, 23155-732) | |