Search by Drug Name or NDC
NDC 23710-0100-70 ECOZA 10 mg/g Details
ECOZA 10 mg/g
ECOZA is a TOPICAL AEROSOL, FOAM in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Exeltis USA Dermatology, LLC. The primary component is ECONAZOLE NITRATE.
MedlinePlus Drug Summary
Econazole is used to treat skin infections such as athlete's foot, jock itch, and ringworm. This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
Related Packages: 23710-0100-70Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Econazole Topical
Product Information
| NDC | 23710-0100 |
|---|---|
| Product ID | 23710-100_d488dcc9-a064-2814-e053-2995a90ac26a |
| Associated GPIs | 90154035103910 |
| GCN Sequence Number | 071812 |
| GCN Sequence Number Description | econazole nitrate FOAM 1 % TOPICAL |
| HIC3 | Q5F |
| HIC3 Description | TOPICAL ANTIFUNGALS |
| GCN | 35806 |
| HICL Sequence Number | 016904 |
| HICL Sequence Number Description | ECONAZOLE NITRATE |
| Brand/Generic | Brand |
| Proprietary Name | ECOZA |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | econazole nitrate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | AEROSOL, FOAM |
| Route | TOPICAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/g |
| Substance Name | ECONAZOLE NITRATE |
| Labeler Name | Exeltis USA Dermatology, LLC |
| Pharmaceutical Class | Azole Antifungal [EPC], Azoles [CS] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA205175 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 23710-0100-70 (23710010070)
| NDC Package Code | 23710-100-70 |
|---|---|
| Billing NDC | 23710010070 |
| Package | 1 CANISTER in 1 CARTON (23710-100-70) / 70 g in 1 CANISTER |
| Marketing Start Date | 2020-10-02 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 998798bc-5c64-4e4c-a0d6-925278ff76a4 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
ECOZA ® (econazole nitrate) topical foam, 1%, for topical use
Initial U.S. Approval: 1982
INDICATIONS AND USAGE
Ecoza is an azole antifungal indicated for the treatment of interdigital tinea pedis caused by Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum in patients 12 years of age and older. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Foam, 1%. ( 3)
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
Contents are flammable. Instruct the patient to avoid heat, flame, and/or smoking during and immediately following application. ( 5.1)
ADVERSE REACTIONS
During clinical trials with Ecoza topical foam, the most common adverse reactions were application site reactions which occurred in less than 1% of subjects in both the Ecoza and vehicle arms. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Quinnova Pharmaceuticals at 1-877-660-6263 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2020
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Flammability
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Warfarin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/ STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
5 WARNINGS AND PRECAUTIONS
5.1 Flammability
Ecoza topical foam is flammable. Avoid heat, flame, and smoking during and immediately following application. Contents under pressure. Do not puncture and/or incinerate the containers. Do not expose containers to heat and/or store at temperatures above 120°F (49°C) even when empty. Do not store in direct sunlight.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two double-blind, vehicle-controlled clinical trials, 495 subjects were exposed to Ecoza topical foam or vehicle (246 subjects were exposed to Ecoza topical foam, 1% and 249 were exposed to vehicle). Subjects with interdigital tinea pedis applied foam or vehicle once daily for approximately 28 days. During clinical trials with Ecoza topical foam, the most common adverse reactions were application site reactions which occurred in less than 1% of subjects in both the Ecoza and vehicle arms.
7 DRUG INTERACTIONS
7.1 Warfarin
Concomitant administration of econazole and warfarin has resulted in enhancement of anticoagulant effect. Most cases reported product application with use under occlusion, genital application, or application to a large body surface area which may increase the systemic absorption of econazole nitrate. Monitoring of International Normalized Ratio (INR) and/or prothrombin time may be indicated especially for patients who apply econazole to large body surface areas, in the genital area, or under occlusion.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled trials with Ecoza topical foam in pregnant women. Ecoza topical foam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Econazole nitrate has not been shown to be teratogenic when administered orally to mice, rabbits or rats. Fetotoxic or embryotoxic effects were observed in Segment I oral studies with rats receiving 10 to 40 times the human dermal dose. Similar effects were observed in Segment II or Segment III studies with mice, rabbits and/or rats receiving oral doses 80 or 40 times the human dermal dose.
8.3 Nursing Mothers
It is not known whether econazole nitrate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when econazole nitrate is administered to a nursing woman. Following oral administration of econazole nitrate to lactating rats, econazole and/or metabolites were excreted in milk and were found in nursing pups.
8.4 Pediatric Use
Of the 173 subjects treated with Ecoza topical foam, 1% in the clinical trials, 2 subjects were 12 to 17 years old. In a pediatric maximal use trial, Ecoza topical foam, 1% was applied once daily to eighteen subjects aged 12 to 17 years with interdigital tinea pedis for 28 days [ see Clinical Pharmacology (12.3)]. The safety findings for subjects 12 to 17 years were similar to those in adult population.
11 DESCRIPTION
Ecoza (econazole nitrate) topical foam, 1% contains the azole antifungal agent, econazole nitrate in an oil-in-water emulsion base consisting of the following inactive ingredients: dimethicone, glycerin, polysorbate 20, povidone, propylene glycol, stearic acid, trolamine, purified water and butane as a propellant. Each gram of Ecoza topical foam, 1% contains 10 mg of econazole nitrate, USP, in a white to off-white foam. Ecoza topical foam, 1% is alcohol (ethanol)-free and for topical use only.
Chemically, econazole nitrate is 1-[2-{(4-chloro-phenyl)methoxy}-2-(2,4-dichlorophenyl) ethyl]-1H-imidazole mononitrate. Econazole nitrate has the molecular formula C 18H 15Cl 3N 2O. HNO 3 and a molecular weight of 444.70. Its molecular structure is as follows:

12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ecoza topical foam is an azole antifungal [ see Clinical Pharmacology (12.4)] .
12.3 Pharmacokinetics
The systemic absorption of Ecoza topical foam, 1% following topical application was studied in one clinical trial in adults and one clinical study in pediatric subjects.
In the adult trial, 19 subjects (male and female) with tinea pedis applied Ecoza topical foam, 1% once daily for 29 days. Subjects applied a mean daily amount of 2.4 g of Ecoza topical foam, 1% to soles, toes, interdigital spaces and tops of both feet up to the ankles. Blood samples were obtained on Day 29 at pre-dose and 1, 2, 4, 6, 8, and 12 hours after application. Results (mean ± SD) showed the time to reach peak plasma concentrations (T max) was 6.8 ± 5.1 h with maximum concentration (C max) of 417 ± 218 pg/ml. The area under the concentration time curve for the first 12 hours post application on Day 29 (AUC (0-12)) was 3440 ± 1920 pg-h/ml.
In the pediatric trial, 18 subjects (male and female ages 12 - 17) with interdigital tinea pedis and positive fungal cultures were treated with Ecoza topical foam, 1% once daily for 4 weeks. Subjects applied a mean daily amount of 3.2 g of Ecoza topical foam, 1% to soles, toes, interdigital spaces and tops of both feet up to the ankles. Blood samples were obtained on Day 28 at pre-dose and 7 h and 11 h post-dose. The mean ± SD econazole plasma concentration was 397 ± 289, 534 ± 745 and 575 ± 638 pg/mL at pre-dose and 7 h and 11 h post-dose, respectively.
12.4 Microbiology
Mechanism of Action
Econazole nitrate, an azole antifungal agent, inhibits fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylase enzyme. This enzyme functions to convert lanosterol to ergosterol. The accumulation of 14 alpha-methyl sterols correlates with the subsequent loss of ergosterol in the fungal cell wall and may be responsible for the fungistatic activity of econazole. Mammalian cell demethylation is less sensitive to econazole inhibition.
Activity in vitro and in clinical infections
Econazole nitrate has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections [ see Indications and Usage (1)].
Trichophyton rubrum
Epidermophyton floccosum
Trichophyton mentagrophytes
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
In two multi-center, randomized, double-blind, vehicle-controlled clinical trials a total of 505 subjects with interdigital tinea pedis were randomized 1:1 to Ecoza topical foam or vehicle; subjects applied the assigned medication once daily for 4 weeks. The severity of erythema, scaling, fissuring, maceration, vesiculation, and pruritus were graded using a 4-point scale (none, mild, moderate, severe). Subjects had KOH examination and fungal cultures taken to confirm eligibility. A total of 339 subjects with positive fungal cultures were evaluated for efficacy. Efficacy was evaluated on Day 43, 2 weeks post-treatment with treatment success being defined as complete cure (negative KOH and fungal culture and no evidence of clinical disease). The study population ranged in age from 12 to 71 years with 5 subjects less than 18 years of age at baseline. The subjects were 71% male and 51% Caucasian. Table 1 presents the efficacy results for each trial.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| Ecoza topical foam, 1%
N = 82 n(%) | Foam Vehicle
N = 83 n(%) | Ecoza topical foam, 1%
N = 91 n(%) | Foam Vehicle
N = 83 n(%) |
|
| Complete cure* | 19 (23.2%) | 2 (2.4%) | 23 (25.3%) | 4 (4.8%) |
| Effective treatment† | 40 (48.8%) | 9 (10.8%) | 44 (48.4%) | 9 (10.8%) |
| Mycological cure‡ | 56 (68.3%) | 13 (15.7%) | 61 (67.0%) | 15 (18.1%) |
16 HOW SUPPLIED/ STORAGE AND HANDLING
Ecoza topical foam, 1% is white to off-white foam supplied in 10g (NDC 23710-100-10) and 70g (NDC 23710-100-75) aluminum pressurized canister.
Store at controlled room temperature 20°C to 25°C (68°F to 77°F) with excursions permitted between 15°C and 30°C (59°F and 86°F). Do not refrigerate or freeze.
Ecoza topical foam is flammable. Avoid heat, flame, and smoking during and immediately following application.
Contents under pressure. Do not puncture and/or incinerate the containers.
Do not expose containers to heat and/or store at temperatures above 120°F (49°C) even when empty.
Do not store in direct sunlight.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
The patient should be instructed as follows:
- Inform patients that Ecoza (econazole nitrate) topical foam, 1% is for topical use only. Ecoza (econazole nitrate) topical foam, 1% is not intended for oral, intravaginal, or ophthalmic use.
- Ecoza topical foam, 1% is flammable; avoid heat, flame, and smoking during and immediately following application.
- If a reaction suggesting sensitivity or chemical irritation develops with the use of Ecoza topical foam, 1%, use of the medication should be discontinued.
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
| Patient Information
ECOZA ® (ee-ko-zah) (econazole nitrate) topical foam, 1% |
||
|---|---|---|
| Important information: Ecoza topical foam is for use on skin only. Do not use Ecoza topical foam in your eyes or vagina. | ||
| What is Ecoza topical foam?
Ecoza topical foam is a prescription medicine used on the skin (topical) to treat athlete's foot that is between the toes (interdigital tinea pedis) in people 12 years of age and older. |
||
| What should I tell my doctor before using Ecoza topical foam?
Before using Ecoza topical foam, tell your doctor about all of your medical conditions, including if you:
|
||
| How should I use Ecoza topical foam?
See the detailed Instructions for Use for information about how to use Ecoza topical foam.
|
||
What should I avoid while using Ecoza topical foam?
|
||
| What are the possible side effects of Ecoza topical foam?
Ecoza topical foam may cause skin reactions at the treatment site. Tell your doctor if you have any skin reactions on the areas of your skin treated with Ecoza topical foam. These are not all the possible side effects of Ecoza topical foam. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
How should I store Ecoza topical foam?
|
||
| General information about the safe and effective use of Ecoza topical foam
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your doctor or pharmacist for information about Ecoza topical foam that is written for health professionals. Do not use Ecoza topical foam for a condition for which it was not prescribed. Do not give Ecoza topical foam to other people, even if they have the same symptoms that you have. It may harm them. |
||
| What are the ingredients in Ecoza topical foam?
Active ingredient: econazole nitrate USP Inactive Ingredients: dimethicone, glycerin, polysorbate 20, povidone, propylene glycol, stearic acid, trolamine, purified water and butane as a propellant. Manufactured for Exeltis USA Dermatology, LLC Florham Park, NJ 07932 For more information call Exeltis USA, Inc. at 1-877-324-9349. |
||
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Issued:07/2016 | 1007501-02 |
Instructions for Use ECOZA ® (ee-ko-zah) (econazole nitrate) topical foam, 1%
| Important information: Ecoza topical foam is for use on skin only.
Do not use Ecoza topical foam in your eyes or vagina. |
Parts of Ecoza topical foam Canister. (See Figure A)
How to apply Ecoza topical foam:
| Step 1: | Before you apply Ecoza topical foam, shake the Ecoza topical foam canister for about 5 seconds. |
| Step 2: | Remove the cap and turn the Ecoza topical foam canister upside down over the palm of your hand. |
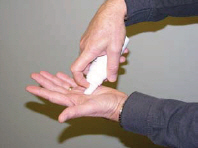
| Step 3: | Press down firmly on the actuator until there is a small amount of foam about the size of a golf ball in the palm of your hand. (See Figures B and C) |
| Step 4: | Use your finger-tips to scoop up small amounts of Ecoza topical foam and apply to the affected skin areas on your feet. Gently rub the foam into the skin. (See Figure D) |
| Figure D | |
| Step 5: | You should apply Ecoza topical foam to your toes, to the spaces between your toes, and to the surrounding areas 1 time a day for 4 weeks or as prescribed by your doctor. |
| Step 6: | Replace the cap. Wash your hands after applying Ecoza topical foam. |
How should I store Ecoza topical foam?
- Store Ecoza topical foam at room temperature, between 68°F to 77°F (20°C to 25°C).
- Do not refrigerate or freeze Ecoza topical foam.
- Do not store Ecoza topical foam in direct sunlight.
- Ecoza topical foam is flammable. Keep the Ecoza topical foam canister away from heat and temperatures above 120°F (49°C), even if the canister is empty.
- Do not puncture or burn the Ecoza topical foam canister.
Keep Ecoza topical foam and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for Exeltis USA Dermatology, LLC Florham Park, NJ 07932
Issued: 07/2016
PRINCIPAL DISPLAY PANEL - 70 g Canister Label
PRINCIPAL DISPLAY PANEL - 70 g Canister Carton
INGREDIENTS AND APPEARANCE
| ECOZA
econazole nitrate aerosol, foam |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Exeltis USA Dermatology, LLC (078715346) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Laboratories, Ltd. | 832224526 | analysis(23710-100) , label(23710-100) , manufacture(23710-100) , pack(23710-100) | |