Search by Drug Name or NDC
NDC 36800-0416-58 topcare triple antibiotic 400; 3.5; 5000 [USP'U]/g; mg/g; [USP'U]/g Details
topcare triple antibiotic 400; 3.5; 5000 [USP'U]/g; mg/g; [USP'U]/g
topcare triple antibiotic is a TOPICAL OINTMENT in the HUMAN OTC DRUG category. It is labeled and distributed by Topco Associates LLC. The primary component is BACITRACIN ZINC; NEOMYCIN SULFATE; POLYMYXIN B SULFATE.
MedlinePlus Drug Summary
Neomycin, polymyxin, and bacitracin combination is used to prevent minor skin injuries such as cuts, scrapes, and burns from becoming infected. Neomycin, polymyxin, and bacitracin are in a class of medications called antibiotics. Neomycin, polymyxin, and bacitracin combination works by stopping the growth of bacteria.
Related Packages: 36800-0416-58Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Neomycin, Polymyxin, and Bacitracin Topical
Product Information
| NDC | 36800-0416 |
|---|---|
| Product ID | 36800-416_6a961ec1-d3e2-4d75-9473-7303f17f58cc |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | topcare triple antibiotic |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | bacitracin zinc, neomycin, polymyxin B |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | OINTMENT |
| Route | TOPICAL |
| Active Ingredient Strength | 400; 3.5; 5000 |
| Active Ingredient Units | [USP'U]/g; mg/g; [USP'U]/g |
| Substance Name | BACITRACIN ZINC; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Labeler Name | Topco Associates LLC |
| Pharmaceutical Class | Aminoglycoside Antibacterial [EPC], Aminoglycosides [CS], Decreased Cell Wall Synthesis & Repair [PE], Polymyxin-class Antibacterial [EPC], Polymyxins [CS] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part333B |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
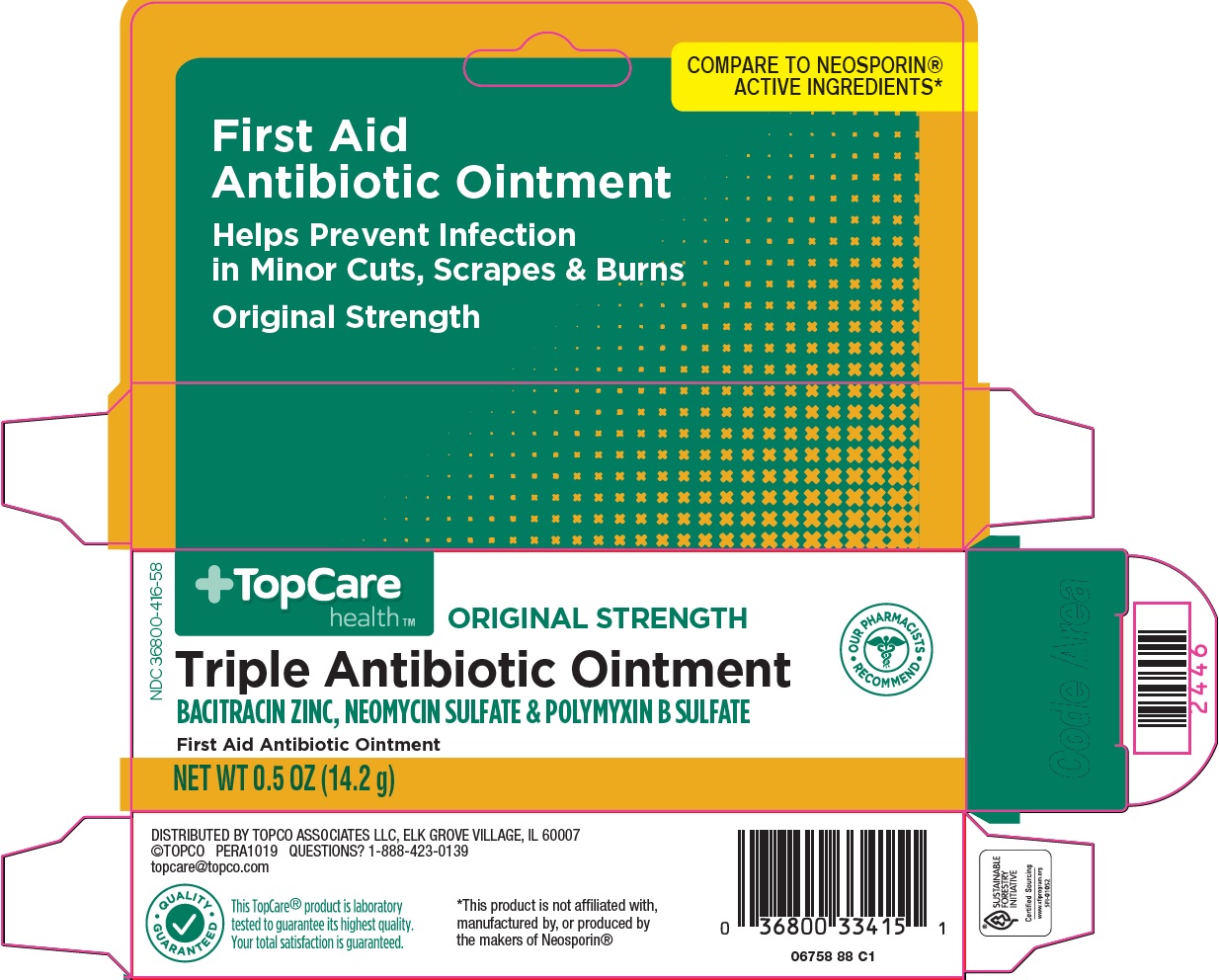

NDC 36800-0416-58 (36800041658)
| NDC Package Code | 36800-416-58 |
|---|---|
| Billing NDC | 36800041658 |
| Package | 1 TUBE in 1 CARTON (36800-416-58) / 14.2 g in 1 TUBE |
| Marketing Start Date | 2020-02-26 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 675665ee-bd0d-4168-81b8-341b1258c102 Details
Active ingredients (in each gram)
Warnings
Directions
Inactive ingredients
Package/Label Principal Display Panel
COMPARE TO NEOSPORIN® ACTIVE INGREDIENTS
First Aid Antibiotic Ointment
Helps Prevent Infection in Minor Cuts, Scrapes & Burns
Original Strength
ORIGINAL STRENGTH
OUR PHARMACISTS RECOMMEND
Triple Antibiotic Ointment
BACITRACIN ZINC, NEOMYCIN SULFATE & POLYMYXIN B SULFATE
First Aid Antibiotic Ointment
NET WT 0.5 OZ (14.2 g)
INGREDIENTS AND APPEARANCE
| TOPCARE TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin, polymyxin b ointment |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Topco Associates LLC (006935977) |