Search by Drug Name or NDC
NDC 36800-0500-02 Severe Allergy Plus Sinus Headache 325; 25; 5 mg/1; mg/1; mg/1 Details
Severe Allergy Plus Sinus Headache 325; 25; 5 mg/1; mg/1; mg/1
Severe Allergy Plus Sinus Headache is a ORAL TABLET, COATED in the HUMAN OTC DRUG category. It is labeled and distributed by TopCare. The primary component is ACETAMINOPHEN; DIPHENHYDRAMINE HYDROCHLORIDE; PHENYLEPHRINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Acetaminophen is used to relieve mild to moderate pain from headaches, muscle aches, menstrual periods, colds and sore throats, toothaches, backaches, and reactions to vaccinations (shots), and to reduce fever. Acetaminophen may also be used to relieve the pain of osteoarthritis (arthritis caused by the breakdown of the lining of the joints). Acetaminophen is in a class of medications called analgesics (pain relievers) and antipyretics (fever reducers). It works by changing the way the body senses pain and by cooling the body.
Related Packages: 36800-0500-02Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Acetaminophen
Diphenhydramine is used to relieve red, irritated, itchy, watery eyes; sneezing; and runny nose caused by hay fever, allergies, or the common cold. Diphenhydramine is also used to relieve cough caused by minor throat or airway irritation. Diphenhydramine is also used to prevent and treat motion sickness, and to treat insomnia (difficulty falling asleep or staying asleep). Diphenhydramine is also used to control abnormal movements in people who have early stage parkinsonian syndrome (a disorder of the nervous system that causes difficulties with movement, muscle control, and balance) or who are experiencing movement problems as a side effect of a medication. Diphenhydramine will relieve the symptoms of these conditions but will not treat the cause of the symptoms or speed recovery. Diphenhydramine should not be used to cause sleepiness in children. Diphenhydramine is in a class of medications called antihistamines. It works by blocking the action of histamine, a substance in the body that causes allergic symptoms.
Related Packages: 36800-0500-02Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Diphenhydramine
Phenylephrine is used to relieve nasal discomfort caused by colds, allergies, and hay fever. It is also used to relieve sinus congestion and pressure. Phenylephrine will relieve symptoms but will not treat the cause of the symptoms or speed recovery. Phenylephrine is in a class of medications called nasal decongestants. It works by reducing swelling of the blood vessels in the nasal passages.
Related Packages: 36800-0500-02Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Phenylephrine
Product Information
| NDC | 36800-0500 |
|---|---|
| Product ID | 36800-500_d0e83438-5412-a1a3-e053-2995a90a8be4 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Severe Allergy Plus Sinus Headache |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | ACETAMINOPHEN, DIPHENHYDRAMINE HYDROCHLORIDE, and PHENYLEPHRINE HYDROCHLORIDE |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, COATED |
| Route | ORAL |
| Active Ingredient Strength | 325; 25; 5 |
| Active Ingredient Units | mg/1; mg/1; mg/1 |
| Substance Name | ACETAMINOPHEN; DIPHENHYDRAMINE HYDROCHLORIDE; PHENYLEPHRINE HYDROCHLORIDE |
| Labeler Name | TopCare |
| Pharmaceutical Class | Adrenergic alpha1-Agonists [MoA], Histamine H1 Receptor Antagonists [MoA], Histamine-1 Receptor Antagonist [EPC], alpha-1 Adrenergic Agonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Listing Certified Through | n/a |
Package
Package Images
NDC 36800-0500-02 (36800050002)
| NDC Package Code | 36800-500-02 |
|---|---|
| Billing NDC | 36800050002 |
| Package | 2 BLISTER PACK in 1 CARTON (36800-500-02) / 10 TABLET, COATED in 1 BLISTER PACK |
| Marketing Start Date | 2013-07-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 8722c9e2-21bb-4772-bdb7-de8687de0a7b Details
SPL UNCLASSIFIED SECTION
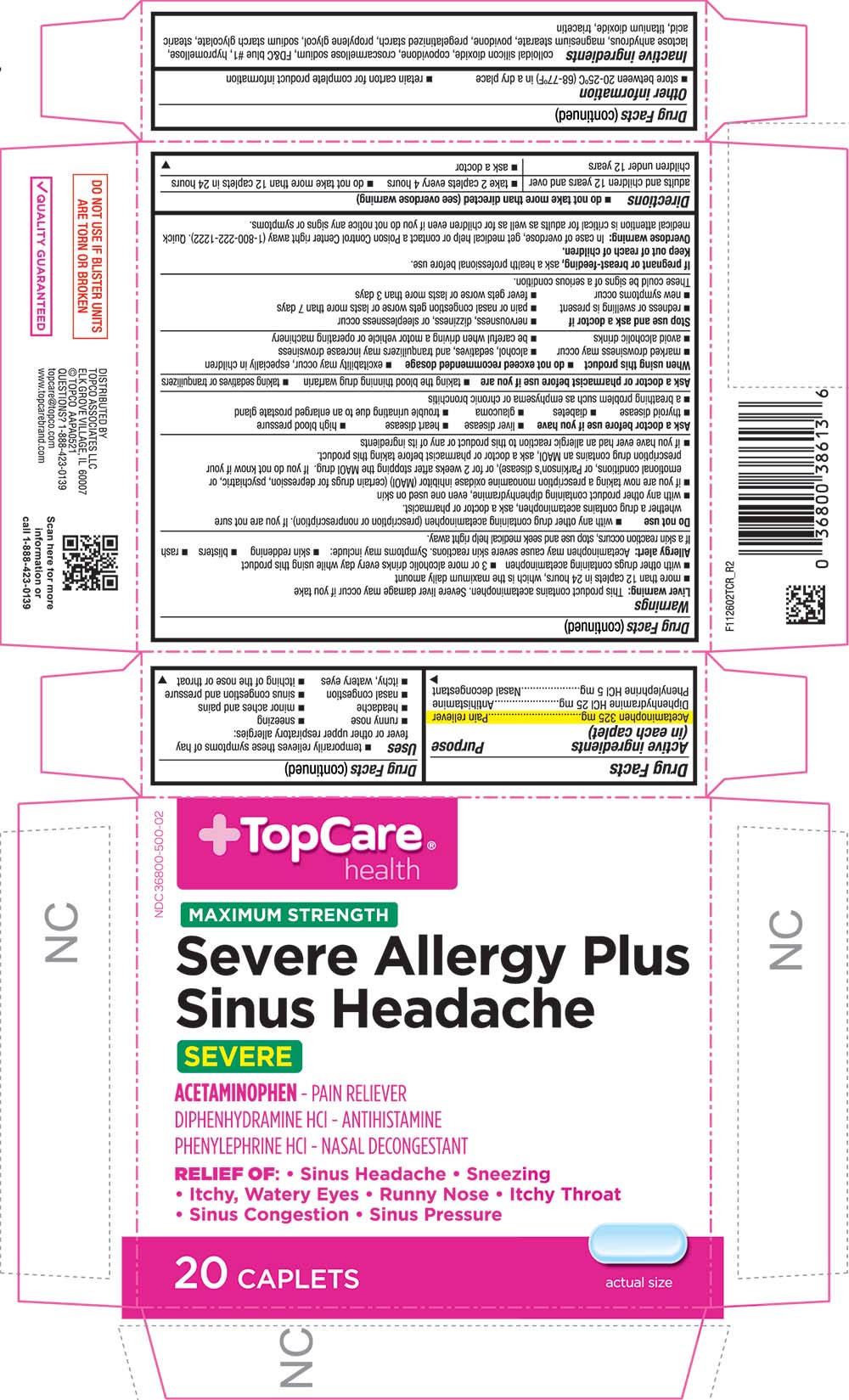
Uses
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 12 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert
acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under 12 years |
|
Other information
Inactive ingredients
PRINCIPAL DISPLAY PANEL
NDC 36800-500-02
TopCare health
MAXIMUM STRENGTH
Severe Allergy Plus Sinus Headache
ACETAMINOPHEN - PAIN RELIEVER
DIPHENHYDRAMINE HCL - ANTIHISTAMINE
PHENYLEPHRINE HCL - NASAL DECONGESTANT
RELIEF OF:
Sinus Headache
Sneezing
Itchy, Watery Eyes
Runny Nose
Itchy Throat
Sinus Congestion
Sinus Pressure
20 CAPLETS
actual size

INGREDIENTS AND APPEARANCE
| SEVERE ALLERGY PLUS SINUS HEADACHE
acetaminophen, diphenhydramine hydrochloride, and phenylephrine hydrochloride tablet, coated |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - TopCare (006935977) |